
Which of the compounds shown in figure react with aniline to give acetanilide?
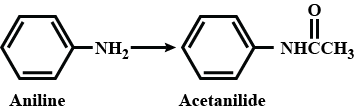
A.\[C{{H}_{3}}COCl\]
B.
C.\[C{{H}_{3}}CHO\]
D.



Answer
568.8k+ views
Hint: Aniline is a primary amine (i.e., one of the hydrogen of ammonia is replaced by benzene) and it can react with acetic anhydride and acetanilide by the reaction called acetylation. Acetylation is a nucleophilic substitution reaction. In nucleophilic substitution reactions, the electronegative compound replaces the existing compound.
Complete answer:
To understand the above reaction, we first need to understand what are nucleophiles, what nucleophilic reactions are and what are primary amines.
First, we will understand what nucleophiles are. The word phile means loving. Nucleophiles are the compounds that are nucleus loving. This means that these compounds themselves are electron rich. Examples of nucleophiles are: Halogens (Iodine, Bromine, Chlorine), hydroxide ion, cyanide ion etc.
Another example of nucleophile is ammonia, as it contains lone pairs and can readily give its electrons to an atom.
Now the nucleophilic substitution reaction occurs when the more electronegative element or compound replaces the less electronegative compound.
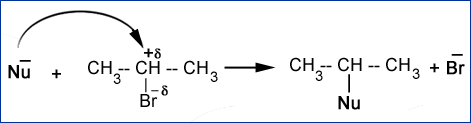
For example, in above equation if we add use hydroxide (\[O{{H}^{-}}\]) as the nucleophile, hydroxide being more electronegative than \[B{{r}^{-}}\]replaces its place.
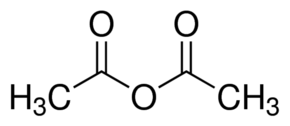
In compounds like acetic anhydride, the delocalisation of electrons in their resonance structure makes it a good electrophile and the lone pair present in amine shares its electrons with acetic anhydride to form acetanilide. Therefore, option b is correct.

The above reaction follows following mechanism:
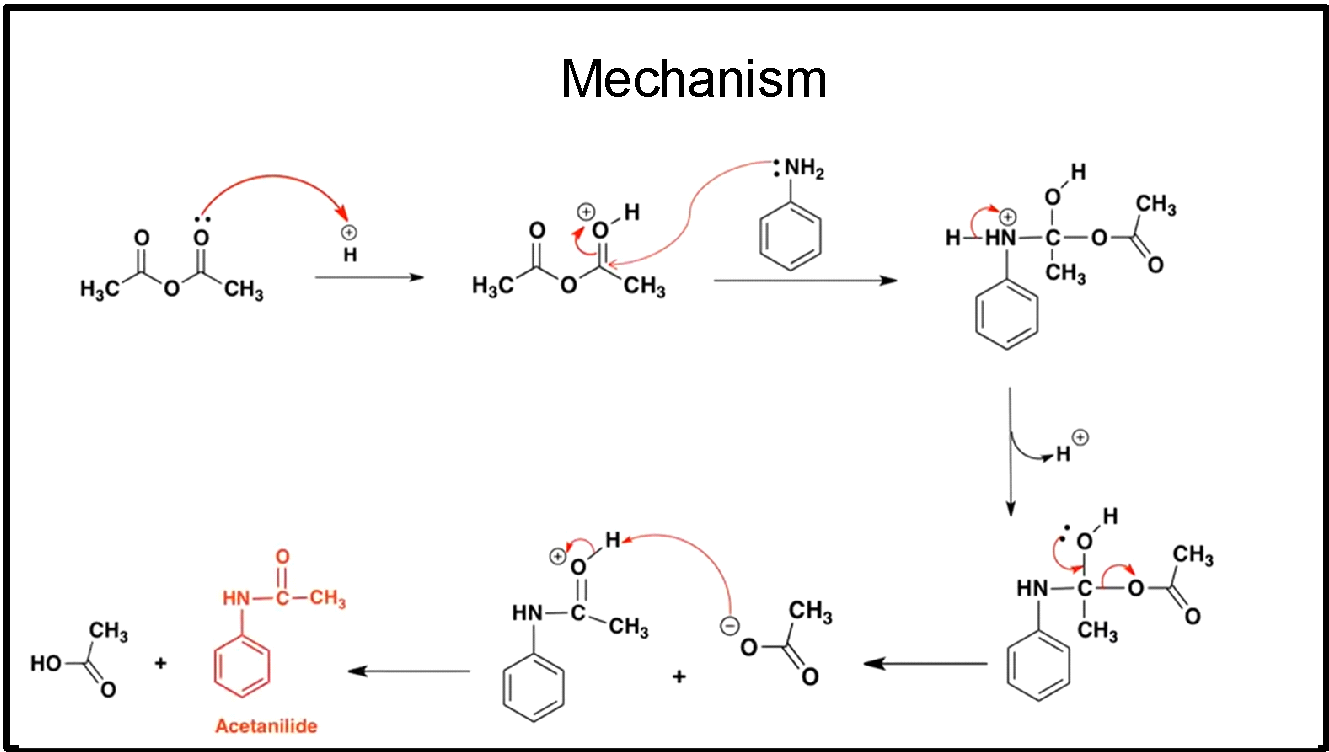
The acetic anhydride, due to the delocalisation of electrons, gains a proton easily and becomes a good electrophile.
It further combines with the lone pair present in amine according to the mechanism shown in above diagram.
Note:
It is important to note that in nucleophilic substitution reactions, the leaving group is less negative and the nucleophile is more electronegative. The nature of the leaving group decides the type of substitution reaction that will occur. These reactions occur in saturated compounds and not in unsaturated compounds.
Complete answer:
To understand the above reaction, we first need to understand what are nucleophiles, what nucleophilic reactions are and what are primary amines.
First, we will understand what nucleophiles are. The word phile means loving. Nucleophiles are the compounds that are nucleus loving. This means that these compounds themselves are electron rich. Examples of nucleophiles are: Halogens (Iodine, Bromine, Chlorine), hydroxide ion, cyanide ion etc.
Another example of nucleophile is ammonia, as it contains lone pairs and can readily give its electrons to an atom.
Now the nucleophilic substitution reaction occurs when the more electronegative element or compound replaces the less electronegative compound.

For example, in above equation if we add use hydroxide (\[O{{H}^{-}}\]) as the nucleophile, hydroxide being more electronegative than \[B{{r}^{-}}\]replaces its place.
In compounds like acetic anhydride, the delocalisation of electrons in their resonance structure makes it a good electrophile and the lone pair present in amine shares its electrons with acetic anhydride to form acetanilide. Therefore, option b is correct.

The above reaction follows following mechanism:

The acetic anhydride, due to the delocalisation of electrons, gains a proton easily and becomes a good electrophile.
It further combines with the lone pair present in amine according to the mechanism shown in above diagram.
Note:
It is important to note that in nucleophilic substitution reactions, the leaving group is less negative and the nucleophile is more electronegative. The nature of the leaving group decides the type of substitution reaction that will occur. These reactions occur in saturated compounds and not in unsaturated compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




