
Which molecule can exhibit keto-enol tautomerism?
(A) Acetaldehyde
(B) Propanol
(C) Propane
(D) Butane
Answer
493.8k+ views
Hint: Remember whatever you have studied about tautomerism. There is the transfer of a proton. In keto-enol tautomerism, a ketone or an aldehyde group is converted to an enol or alcohol group. Check the structures of all the molecules. They should have alpha hydrogen in them to undergo keto-enol tautomerism.
Complete Step By Step Answer:
Tautomers are isomers of a compound that differ only in the position of the protons and electrons. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism.
Keto–enol tautomerism refers to a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol (alcohol). The keto and enol forms are said to be tautomers of each other.
Now we need to find which among the above options can exhibit keto-enol tautomerism. For finding out the solution we must know the structures of all the compounds. So the structures are as follows:
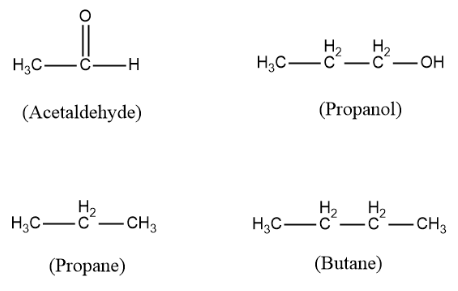
For a compound to exhibit keto-enol tautomerism it should have alpha hydrogen next to a carbonyl group. In the above structures only acetaldehyde has a carbonyl group and all the three options do not have. Acetaldehyde also has an alpha hydrogen in it which is attached to its alpha carbon ( carbon next to the carbonyl group).
Thus, acetaldehyde is the molecule that can exhibit keto-enol tautomerism. It undergoes tautomerism in the following manner:
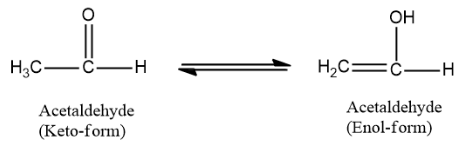
Therefore the correct option is A. acetaldehyde.
Note:
Alpha hydrogen in general is hydrogen attached to alpha carbon that is next to a functional group. So with this definition even propanol has alpha hydrogen but this does not mean it will undergo tautomerism. Keto-enol tautomerism is only exhibited by those molecules having a carbonyl group only. In tautomerism there is transfer of protons and it uses a catalyst for the reaction to take place.
Complete Step By Step Answer:
Tautomers are isomers of a compound that differ only in the position of the protons and electrons. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism.
Keto–enol tautomerism refers to a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol (alcohol). The keto and enol forms are said to be tautomers of each other.
Now we need to find which among the above options can exhibit keto-enol tautomerism. For finding out the solution we must know the structures of all the compounds. So the structures are as follows:

For a compound to exhibit keto-enol tautomerism it should have alpha hydrogen next to a carbonyl group. In the above structures only acetaldehyde has a carbonyl group and all the three options do not have. Acetaldehyde also has an alpha hydrogen in it which is attached to its alpha carbon ( carbon next to the carbonyl group).
Thus, acetaldehyde is the molecule that can exhibit keto-enol tautomerism. It undergoes tautomerism in the following manner:

Therefore the correct option is A. acetaldehyde.
Note:
Alpha hydrogen in general is hydrogen attached to alpha carbon that is next to a functional group. So with this definition even propanol has alpha hydrogen but this does not mean it will undergo tautomerism. Keto-enol tautomerism is only exhibited by those molecules having a carbonyl group only. In tautomerism there is transfer of protons and it uses a catalyst for the reaction to take place.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




