
Which method do you suggest for extraction of high reactivity metals? Why?
Answer
584.4k+ views
Hint: The high reactivity metals being unreactive to carbon are not reduced by it and in their molten state, a pure form can be obtained by separating its ions.
Complete step by step answer:
In nature, the ores are naturally occurring rocks that contain minerals such as metals and other compounds in sufficient amounts. Thus, the metals found in the combined state in the ores are extracted from it, where the extraction method depends upon the reactivity of the metal and stability of its ore. It is generally present as oxides or sulphides in its combined state.
Depending on the reaction of the metals with acids and water, their reactive strength is determined giving us the following activity series. Thus, the reactivity order of a metal with respect to the other metals as follows:
\[\begin{align}
& Li\text{ }Cs>\text{ }Rb>\text{ }K\text{ }>Ba>\text{ }Sr>\text{ }Ca>\text{ }Na>\text{ }Mg>\text{ }Be>\text{ }Al> \\
& C>\text{ }Zn>\text{ }Cr>\text{ }Fe>\text{ }Cd>\text{ }Co>\text{ }Ni>\text{ }Sn>\text{ }Pb>\text{ } \\
& H>\text{ }Sb>\text{ }As>\text{ }Bi>\text{ }Cu>\text{ }Ag>\text{ }Pd>\text{ }Hg>\text{ }Pt>\text{ }Au \\
\end{align}\]
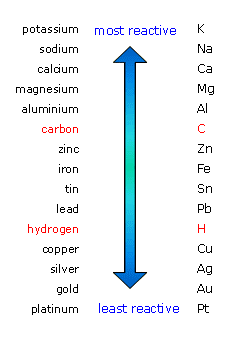
In this series, the metals above carbon are the highly reactive metals and cannot be reduced by carbon. So, for the extraction of these metals from its ores, the electrolysis method is undertaken.
In the extraction process through electrolysis, the electrolytic reduction of the metal from its salt takes place. As the electric current is passed through molten state, which acts as the electrolyte having the ions free to move and the metal being positively charged gets deposited on the cathode. Thus, a pure form of the metal is obtained.
Therefore, the highly reactive metals such as potassium, sodium, calcium, magnesium and aluminium are extracted by electrolysis.
Note: The metals below carbon can be extracted by reduction reaction with carbon as they are less reactive than it and the metals below hydrogen do not need extraction as they are present natively in their pure form.
Complete step by step answer:
In nature, the ores are naturally occurring rocks that contain minerals such as metals and other compounds in sufficient amounts. Thus, the metals found in the combined state in the ores are extracted from it, where the extraction method depends upon the reactivity of the metal and stability of its ore. It is generally present as oxides or sulphides in its combined state.
Depending on the reaction of the metals with acids and water, their reactive strength is determined giving us the following activity series. Thus, the reactivity order of a metal with respect to the other metals as follows:
\[\begin{align}
& Li\text{ }Cs>\text{ }Rb>\text{ }K\text{ }>Ba>\text{ }Sr>\text{ }Ca>\text{ }Na>\text{ }Mg>\text{ }Be>\text{ }Al> \\
& C>\text{ }Zn>\text{ }Cr>\text{ }Fe>\text{ }Cd>\text{ }Co>\text{ }Ni>\text{ }Sn>\text{ }Pb>\text{ } \\
& H>\text{ }Sb>\text{ }As>\text{ }Bi>\text{ }Cu>\text{ }Ag>\text{ }Pd>\text{ }Hg>\text{ }Pt>\text{ }Au \\
\end{align}\]

In this series, the metals above carbon are the highly reactive metals and cannot be reduced by carbon. So, for the extraction of these metals from its ores, the electrolysis method is undertaken.
In the extraction process through electrolysis, the electrolytic reduction of the metal from its salt takes place. As the electric current is passed through molten state, which acts as the electrolyte having the ions free to move and the metal being positively charged gets deposited on the cathode. Thus, a pure form of the metal is obtained.
Therefore, the highly reactive metals such as potassium, sodium, calcium, magnesium and aluminium are extracted by electrolysis.
Note: The metals below carbon can be extracted by reduction reaction with carbon as they are less reactive than it and the metals below hydrogen do not need extraction as they are present natively in their pure form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




