
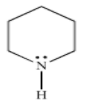
Which is the strongest base?
(A)- Pyrrole
(B)- Aniline
(C)- Pyridine
(D)-

Answer
524.2k+ views
Hint: A compound is a base which can easily donate its electrons to the proton forming a conjugate acid. The value of the $p{{K}_{a}}$of this conjugate acid can determine the strength of basicity of its base.
Complete step by step answer:
In the given compounds, the nitrogen atom has a lone pair of electrons which makes these compounds basic in nature and the order of their basicity can be done on the basis of the ease to share these lone pair of electrons with ${{H}^{+}}$ ions and thus, stability of its conjugate acid formed.
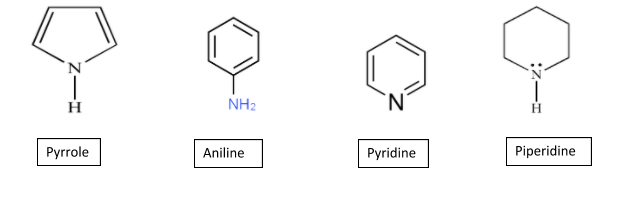
In case of a pyrrole molecule, the lone pair of electrons on the nitrogen atom is in delocalisation with the aromatic ring. So, when it shares these electrons with the proton, it distorts the aromaticity in the ring making it unstable and unfavourable. Thus, have the least tendency to donate its electron pair to the proton.
Whereas, in case of aniline and pyridine molecules, the lone pair on the nitrogen atom are present in the $s{{p}^{2}}$ hybrid orbital of the nitrogen atom due to the $C=N$ bond. So, it does not take part in the delocalisation within the aromatic ring and can easily share its lone pair. But due the presence of the lone pairs in the $s{{p}^{2}}$ orbitals which are strongly attracted to the nucleus make the sharing a little less easy. Thus, slightly basic in nature.
But in the piperidine molecule, these lone pairs are present in the $s{{p}^{3}}$ orbitals of the nitrogen, that is, secondary amine and there is no resonance in the ring. This makes it easy for the nitrogen to share its electrons and is highly basic.
Therefore, the strongest base is option (D)- piperidine.
Note: The stronger base has the highest value of $p{{K}_{a}}$ ,of its conjugate acid which is formed after the base donates its electron to proton.
The order of basicity in the given compounds is:
Piperidine > Aniline > Pyridine > Pyrrole
Complete step by step answer:
In the given compounds, the nitrogen atom has a lone pair of electrons which makes these compounds basic in nature and the order of their basicity can be done on the basis of the ease to share these lone pair of electrons with ${{H}^{+}}$ ions and thus, stability of its conjugate acid formed.

In case of a pyrrole molecule, the lone pair of electrons on the nitrogen atom is in delocalisation with the aromatic ring. So, when it shares these electrons with the proton, it distorts the aromaticity in the ring making it unstable and unfavourable. Thus, have the least tendency to donate its electron pair to the proton.
Whereas, in case of aniline and pyridine molecules, the lone pair on the nitrogen atom are present in the $s{{p}^{2}}$ hybrid orbital of the nitrogen atom due to the $C=N$ bond. So, it does not take part in the delocalisation within the aromatic ring and can easily share its lone pair. But due the presence of the lone pairs in the $s{{p}^{2}}$ orbitals which are strongly attracted to the nucleus make the sharing a little less easy. Thus, slightly basic in nature.
But in the piperidine molecule, these lone pairs are present in the $s{{p}^{3}}$ orbitals of the nitrogen, that is, secondary amine and there is no resonance in the ring. This makes it easy for the nitrogen to share its electrons and is highly basic.
Therefore, the strongest base is option (D)- piperidine.
Note: The stronger base has the highest value of $p{{K}_{a}}$ ,of its conjugate acid which is formed after the base donates its electron to proton.
The order of basicity in the given compounds is:
Piperidine > Aniline > Pyridine > Pyrrole
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




