
Which is the correct thermal stability order of $ {{\text{H}}_2}{\text{E}}\left( {{\text{E = O,S,Se,Te}}{\text{ and Po }}} \right) $ ?
A) $ {{\text{H}}_2}{\text{S < }}{{\text{H}}_2}{\text{O}} < {{\text{H}}_2}{\text{Se < }}{{\text{H}}_2}{\text{Te < }}{{\text{H}}_2}{\text{Po}} $
B) $ {{\text{H}}_2}{\text{O}} < {{\text{H}}_2}{\text{S}} < {{\text{H}}_2}{\text{Se < }}{{\text{H}}_2}{\text{Te < }}{{\text{H}}_2}{\text{Po}} $
C) $ {{\text{H}}_2}{\text{Po < }}{{\text{H}}_2}{\text{Te}} < {{\text{H}}_2}{\text{Se < }}{{\text{H}}_2}{\text{S < }}{{\text{H}}_2}{\text{O}} $
D) $ {{\text{H}}_2}{\text{Se < }}{{\text{H}}_2}{\text{Te}} < {{\text{H}}_2}{\text{Po < }}{{\text{H}}_2}{\text{O < }}{{\text{H}}_2}{\text{S}} $
Answer
537.6k+ views
Hint :All the elements are of Oxygen family .On going down the group, the thermal stability of $ {{\text{H}}_2}{\text{E}} $ decreases as the $ {\text{M - H}} $ bond energy decreases where M represents metals. So, $ {{\text{H}}_2}{\text{O}} $ will be the most thermally stable molecule.
Complete Step By Step Answer:
$ {\text{O,S,Se,Te,Po}} $ are the members of the oxygen family.
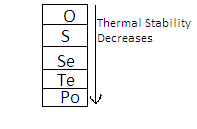
On going down the group, the thermal stability of $ {{\text{H}}_2}{\text{E}} $ decreases where E represents members of the oxygen family. This is because the $ {\text{M - H}} $ bond energy decreases as the size of the central atom increases. On going down the group,
The size of the central atom increases (due to increase in number of electrons).
The electrons of the most outer shell are far distance from the nucleus so they can be easily given which decreases the thermal stability.
The $ {\text{M - H}} $ atoms when combining their orbitals overlap. Thus they form a lower energy state and become stable.
This means that the two atoms come so close they penetrate each other's orbital and a new hybridized orbital is formed which is stable.
So the greater the overlapping, the greater is the bond energy. $ {{\text{H}}_2}{\text{O}} $ has greater overlapping than the other $ {{\text{H}}_2}{\text{E}} $ .
So the order is $ {{\text{H}}_2}{\text{Po < }}{{\text{H}}_2}{\text{Te}} < {{\text{H}}_2}{\text{Se < }}{{\text{H}}_2}{\text{S < }}{{\text{H}}_2}{\text{O}} $ .
Hence the correct answer is ‘C’.
Note :
The $ {\text{M - H}} $ bond energy is the energy required to break the bond formed between the M and H atom. This energy is affected by the following-
1. Atomic radius- with the increase in atomic radius, the bond energy between M and H decreases.
2. Nuclear charge-as the nuclear charge between the nucleus and the outer shell decreases, the bond energy also decreases.
Complete Step By Step Answer:
$ {\text{O,S,Se,Te,Po}} $ are the members of the oxygen family.

On going down the group, the thermal stability of $ {{\text{H}}_2}{\text{E}} $ decreases where E represents members of the oxygen family. This is because the $ {\text{M - H}} $ bond energy decreases as the size of the central atom increases. On going down the group,
The size of the central atom increases (due to increase in number of electrons).
The electrons of the most outer shell are far distance from the nucleus so they can be easily given which decreases the thermal stability.
The $ {\text{M - H}} $ atoms when combining their orbitals overlap. Thus they form a lower energy state and become stable.
This means that the two atoms come so close they penetrate each other's orbital and a new hybridized orbital is formed which is stable.
So the greater the overlapping, the greater is the bond energy. $ {{\text{H}}_2}{\text{O}} $ has greater overlapping than the other $ {{\text{H}}_2}{\text{E}} $ .
So the order is $ {{\text{H}}_2}{\text{Po < }}{{\text{H}}_2}{\text{Te}} < {{\text{H}}_2}{\text{Se < }}{{\text{H}}_2}{\text{S < }}{{\text{H}}_2}{\text{O}} $ .
Hence the correct answer is ‘C’.
Note :
The $ {\text{M - H}} $ bond energy is the energy required to break the bond formed between the M and H atom. This energy is affected by the following-
1. Atomic radius- with the increase in atomic radius, the bond energy between M and H decreases.
2. Nuclear charge-as the nuclear charge between the nucleus and the outer shell decreases, the bond energy also decreases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




