
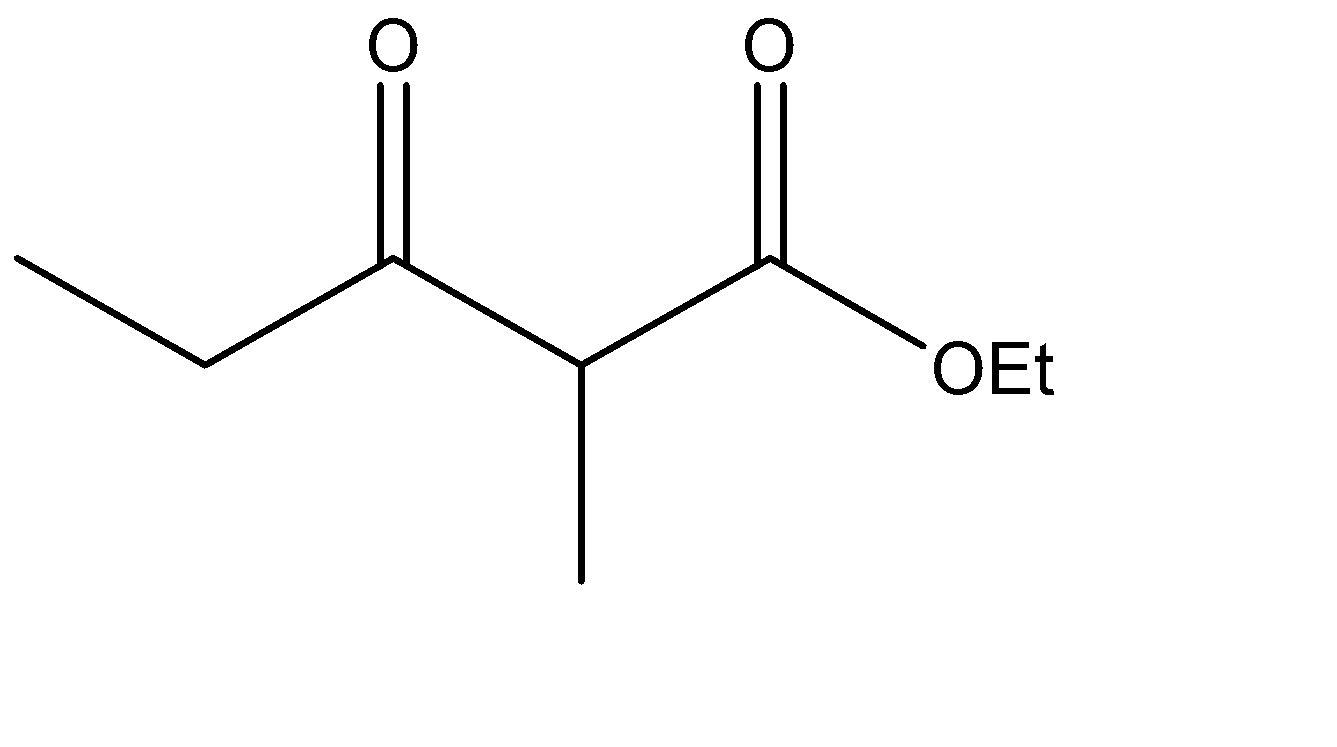
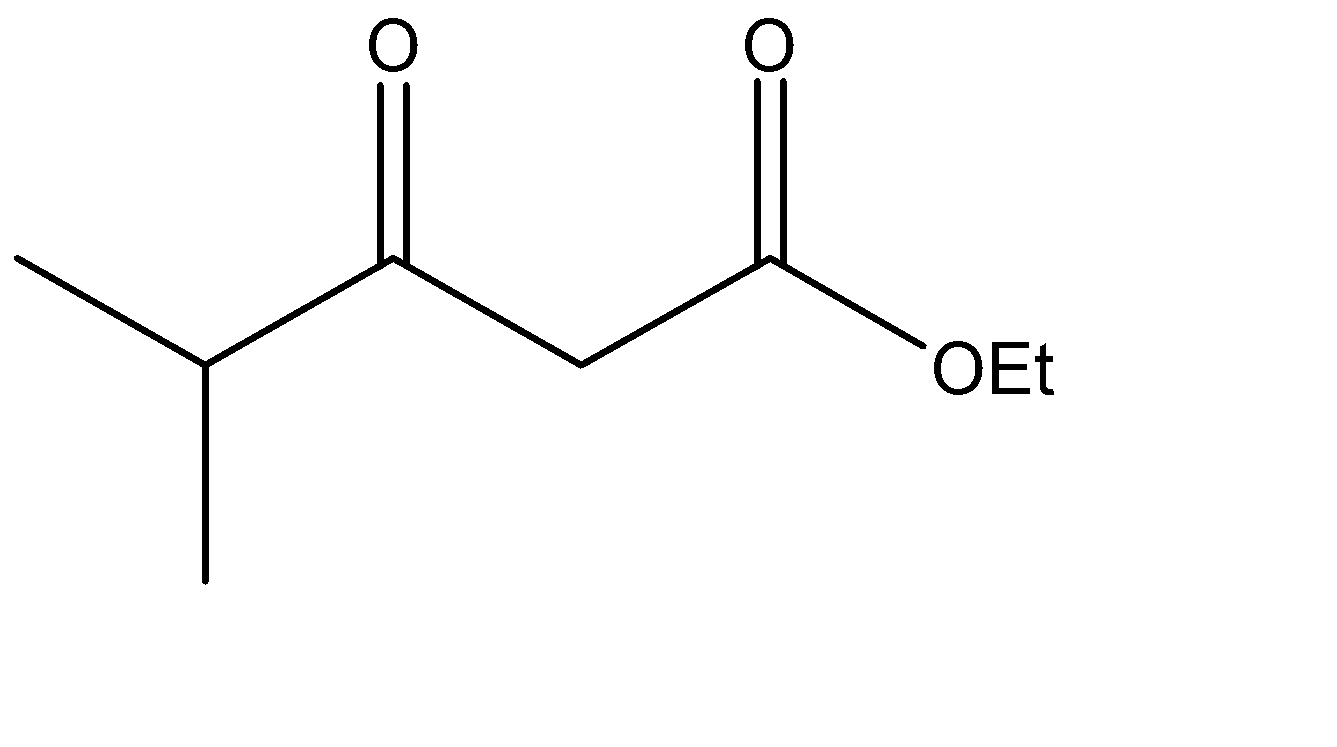
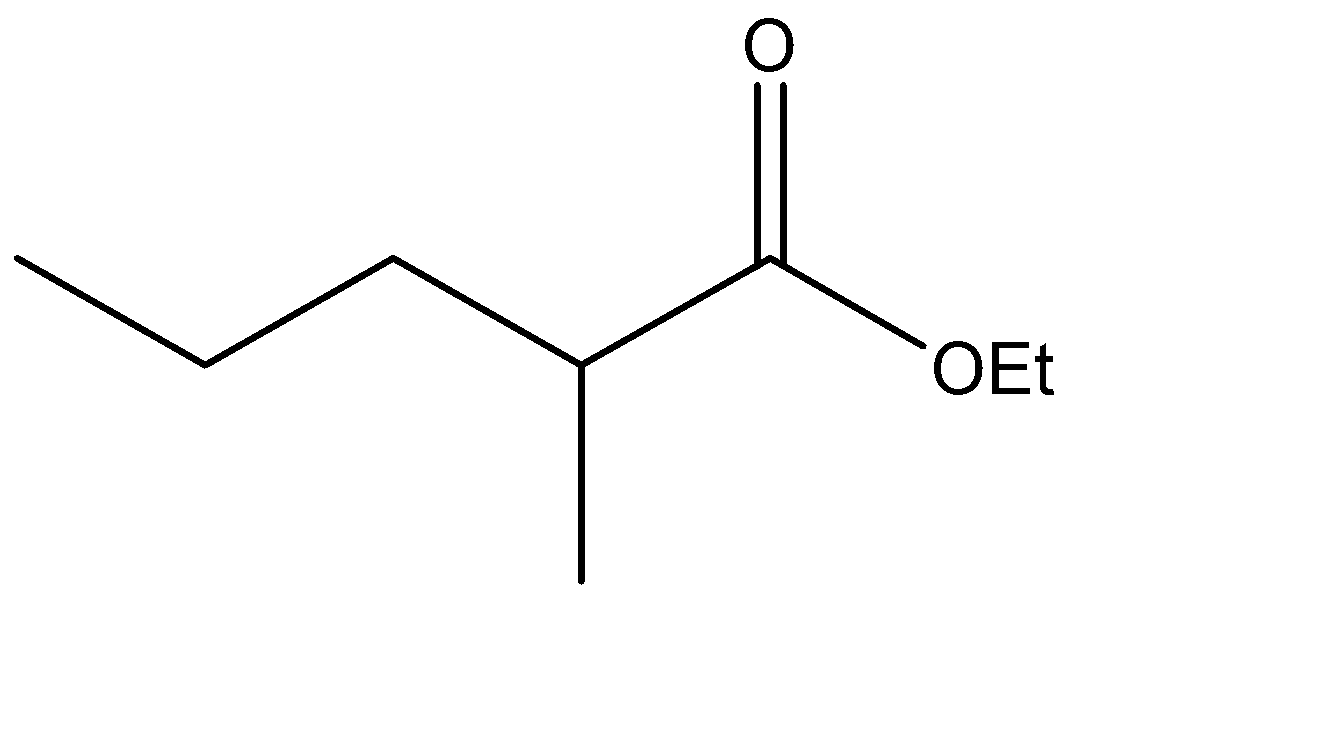
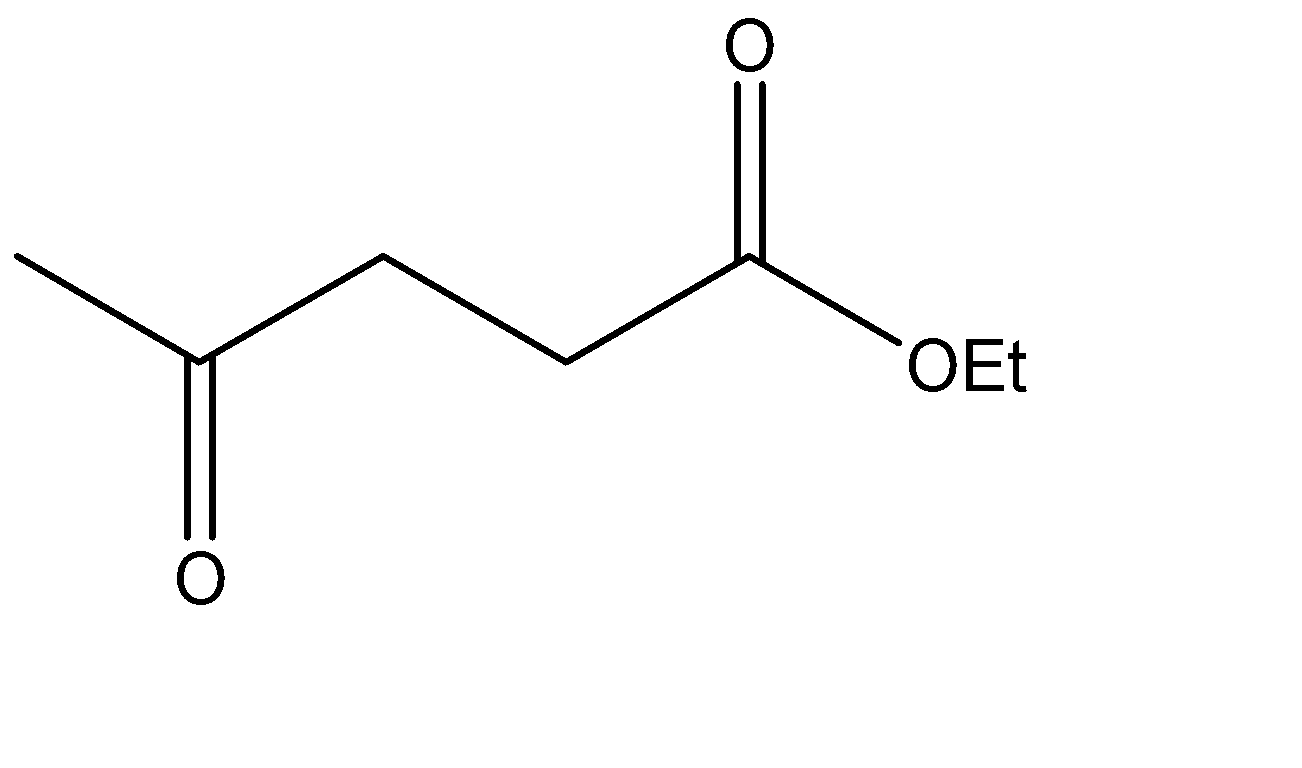
Which is the Claisen condensation product of ethyl propanoate?
A.
B.
C.
D.




Answer
585.9k+ views
Hint: To solve this question, we must first briefly discuss the mechanism and types of products formed in Claisen condensation reaction. Then we must proceed with these steps and try to form the product from the reactant given to us.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The Claisen condensation reaction basically involves the formation of an ester which has ketone functional groups present at the pre – terminal carbon or the beta -carbon atom. Hence, the product formed in Claisen condensation reactions is known as \[\beta \] - keto esters or \[\beta \] - diketone. The Claisen condensation reaction involves the reaction of two esters which undergo a coupling reaction, which eliminates the alkoxy group from one ester and an alpha hydrogen from the other ester. This results in the formation of a new \[C - C\] bond, between the two reacting compounds. It also results in the formation of alcohol from the eliminated alkoxy group. The catalysts used in this reaction are sodium alginate and hydronium ions.
The ester given to us in the question is ethyl propanoate, and it is made to undergo self – condensation. The chemical equation for the same can be given as:
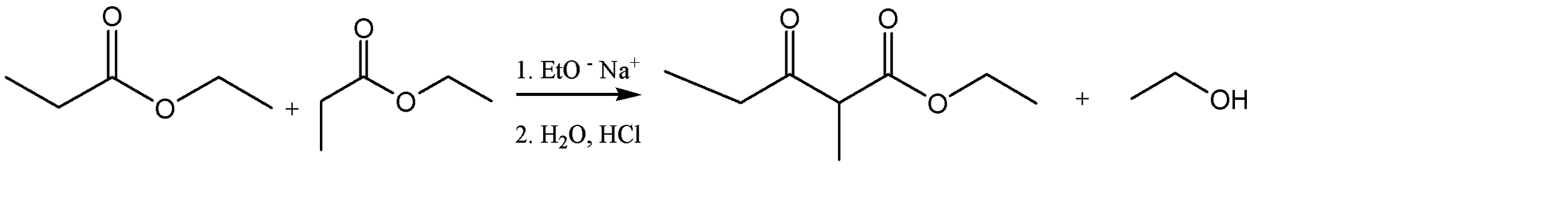
Hence, Option A is the correct option
Note: There are 3 main types of Claisen condensation reactions. The first type includes the self – condensation between two molecules of the same ester. The second type includes two different esters, which may contain similar or different alkyl groups.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The Claisen condensation reaction basically involves the formation of an ester which has ketone functional groups present at the pre – terminal carbon or the beta -carbon atom. Hence, the product formed in Claisen condensation reactions is known as \[\beta \] - keto esters or \[\beta \] - diketone. The Claisen condensation reaction involves the reaction of two esters which undergo a coupling reaction, which eliminates the alkoxy group from one ester and an alpha hydrogen from the other ester. This results in the formation of a new \[C - C\] bond, between the two reacting compounds. It also results in the formation of alcohol from the eliminated alkoxy group. The catalysts used in this reaction are sodium alginate and hydronium ions.
The ester given to us in the question is ethyl propanoate, and it is made to undergo self – condensation. The chemical equation for the same can be given as:

Hence, Option A is the correct option
Note: There are 3 main types of Claisen condensation reactions. The first type includes the self – condensation between two molecules of the same ester. The second type includes two different esters, which may contain similar or different alkyl groups.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




