
Which is not the resonance structure of methyl isocyanate?
A)
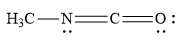
B)
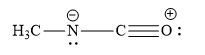
C)
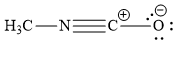
D)
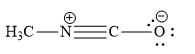
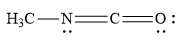
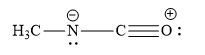
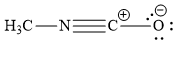
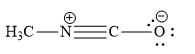
Answer
578.1k+ views
Hint: Resonance structures are the Lewis structures that describe the delocalisation of electrons in a molecule.
To check for resonance structure consider the formal charges on each atom. Also check if each atom has completed its octet.
Complete answer:
The atomic number of carbon is 6. It has 4 valence electrons. It shares its 4 valence electrons with 4 electrons of 4 other atoms to form 4 bonds. When C atom forms 4 bonds, it has no charge and is neutral in nature. When a carbon atom forms only 3 bonds and it has no unshared electron(s), then such carbon atom has unit positive charge. When a carbon atom forms only 3 bonds and it has one unshared electron pair, then such carbon atom has unit negative charge.
In the resonance structure given in the option (C), the carbon atom is surrounded by 8 valence electrons. Yet carbon atoms are shown to have positive charge. This is not possible. Instead of carbon atom, the positive charge should have been on nitrogen atom as shown below:
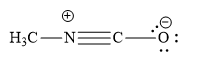
This is because, nitrogen atom forms 3 covalent bonds and one coordinate bond. Nitrogen atoms donate its lone pair of electrons to carbon atoms to form a coordinate bond. In turn, the pi electrons of carbon-oxygen double bonds are given to oxygen atoms. So oxygen atom gains unit negative charge.
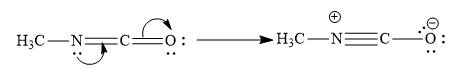
The option (C) represents incorrect resonance structure of methyl isocyanate.
Note: Resonance structures are obtained by movement of pi electrons or unshared electrons. Electrons of sigma bonds do not move. Similarly, the atoms do not move and their position remains fixed.
To check for resonance structure consider the formal charges on each atom. Also check if each atom has completed its octet.
Complete answer:
The atomic number of carbon is 6. It has 4 valence electrons. It shares its 4 valence electrons with 4 electrons of 4 other atoms to form 4 bonds. When C atom forms 4 bonds, it has no charge and is neutral in nature. When a carbon atom forms only 3 bonds and it has no unshared electron(s), then such carbon atom has unit positive charge. When a carbon atom forms only 3 bonds and it has one unshared electron pair, then such carbon atom has unit negative charge.
In the resonance structure given in the option (C), the carbon atom is surrounded by 8 valence electrons. Yet carbon atoms are shown to have positive charge. This is not possible. Instead of carbon atom, the positive charge should have been on nitrogen atom as shown below:

This is because, nitrogen atom forms 3 covalent bonds and one coordinate bond. Nitrogen atoms donate its lone pair of electrons to carbon atoms to form a coordinate bond. In turn, the pi electrons of carbon-oxygen double bonds are given to oxygen atoms. So oxygen atom gains unit negative charge.

The option (C) represents incorrect resonance structure of methyl isocyanate.
Note: Resonance structures are obtained by movement of pi electrons or unshared electrons. Electrons of sigma bonds do not move. Similarly, the atoms do not move and their position remains fixed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




