
Which is not the correct synthesis of m-bromo nitro benzene?
A.
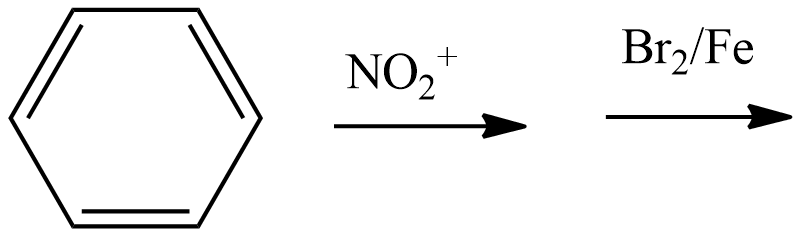
B.
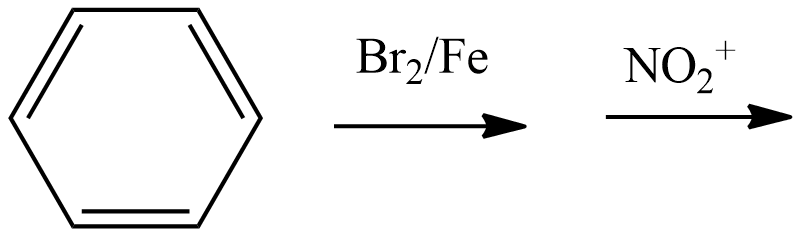
C. Both of these
D. None of these

Answer
512.7k+ views
Hint: The bromine molecule in the presence of the iron is going to act as a para directing group and the nitro group is going to act as a meta directing group for the nucleophilic substitution chemical reactions.
Complete answer:
- In the question is asked to find the correct way for the synthesis of the m-bromo nitro benzene from the given options.
- Coming to the option A,
- We have to write the chemical reaction to know the product which is going to be formed in the above chemical reaction and it is as follows.
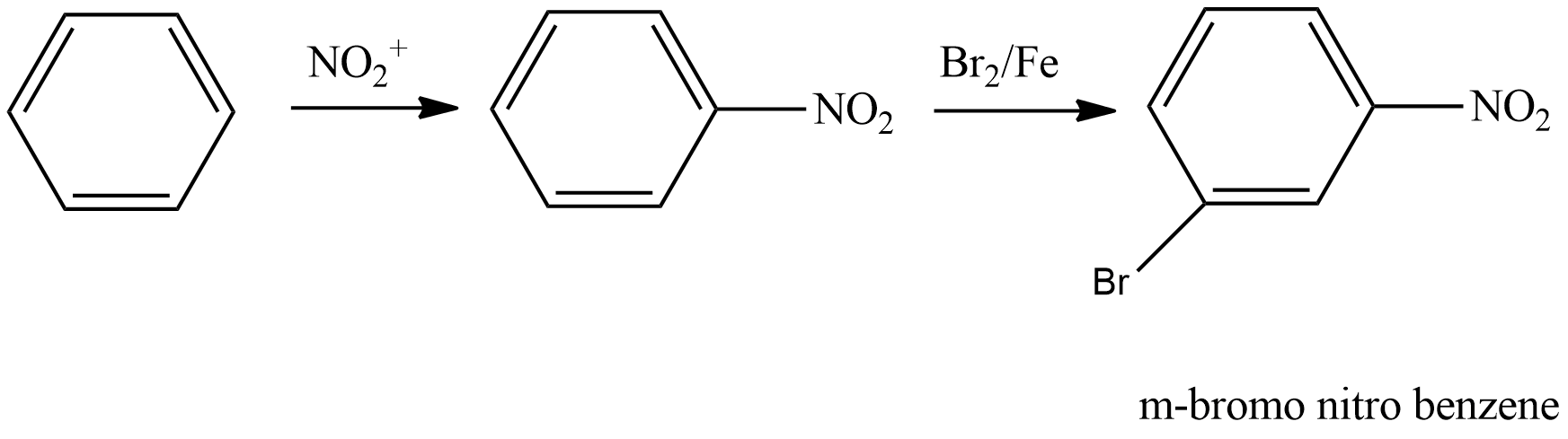
- We know that the nitro group is a meta directing group and it is going to direct the bromine to the meta position and result in the formation of the m-bromo nitro benzene.
- Coming to the option B,

- We have to write the chemical reaction to know the product which is going to be formed in the above chemical reaction and it is as follows.
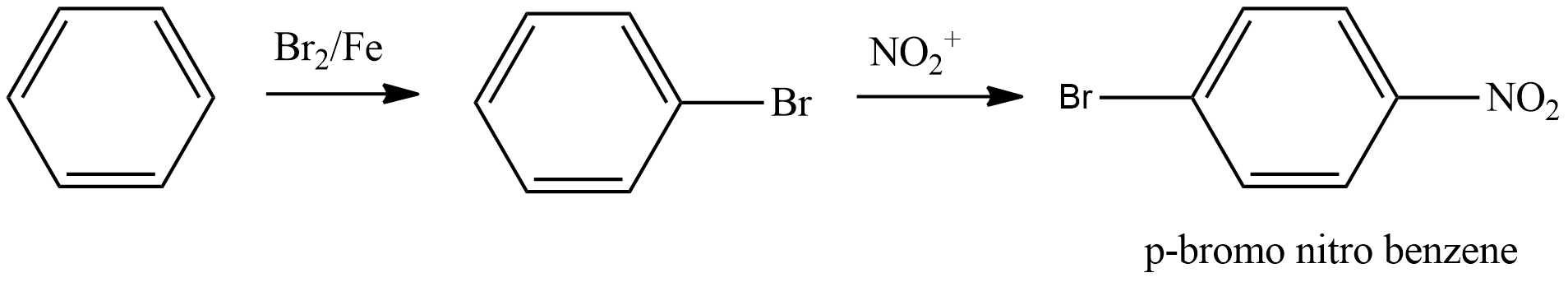
- We know that bromine in the presence of the iron is going to direct the nitro group to the para position.
- Therefore, we can prepare the m-bromo nitro benzene using the chemical reaction which is present in option A.
So, option A is correct.
Note:
The property of the substituent which is present on the benzene ring is going to decide or direct the attachment of the remaining atoms that are going to be involved in the chemical reaction with the substituted benzene ring.
Complete answer:
- In the question is asked to find the correct way for the synthesis of the m-bromo nitro benzene from the given options.
- Coming to the option A,

- We have to write the chemical reaction to know the product which is going to be formed in the above chemical reaction and it is as follows.

- We know that the nitro group is a meta directing group and it is going to direct the bromine to the meta position and result in the formation of the m-bromo nitro benzene.
- Coming to the option B,

- We have to write the chemical reaction to know the product which is going to be formed in the above chemical reaction and it is as follows.

- We know that bromine in the presence of the iron is going to direct the nitro group to the para position.
- Therefore, we can prepare the m-bromo nitro benzene using the chemical reaction which is present in option A.
So, option A is correct.
Note:
The property of the substituent which is present on the benzene ring is going to decide or direct the attachment of the remaining atoms that are going to be involved in the chemical reaction with the substituted benzene ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




