
Which is not the correct reaction?
A.
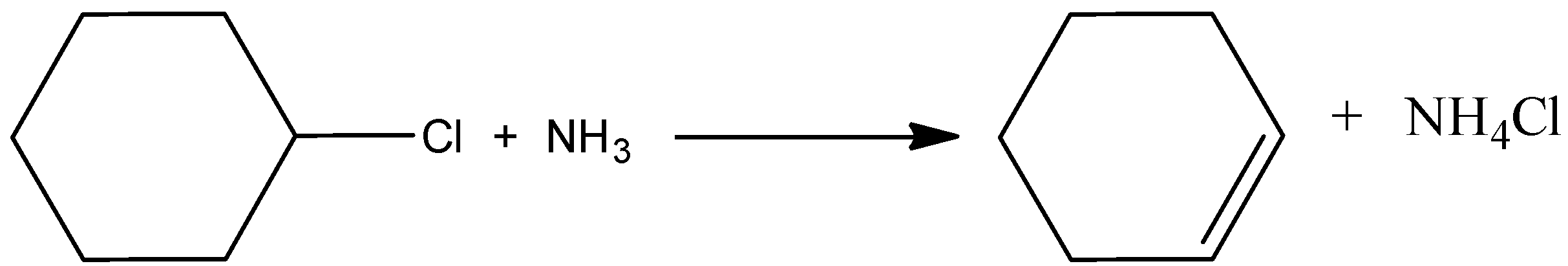
B.
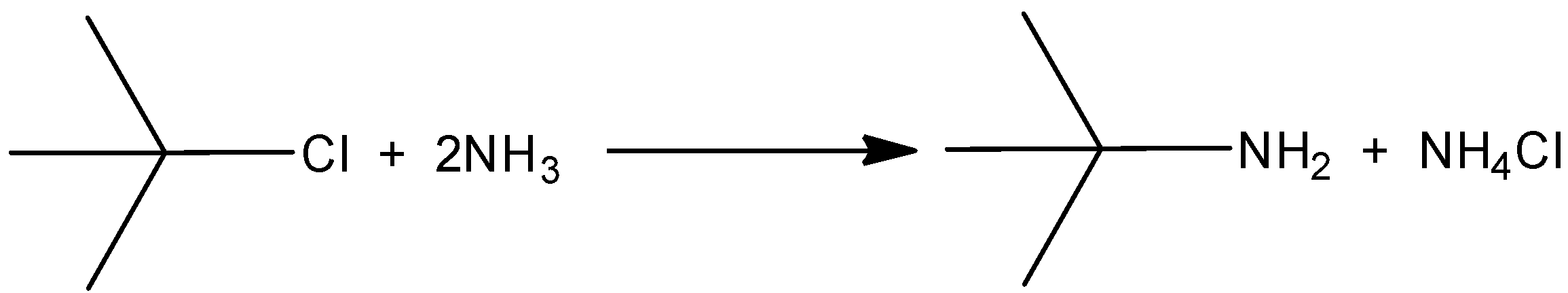
C.

D.
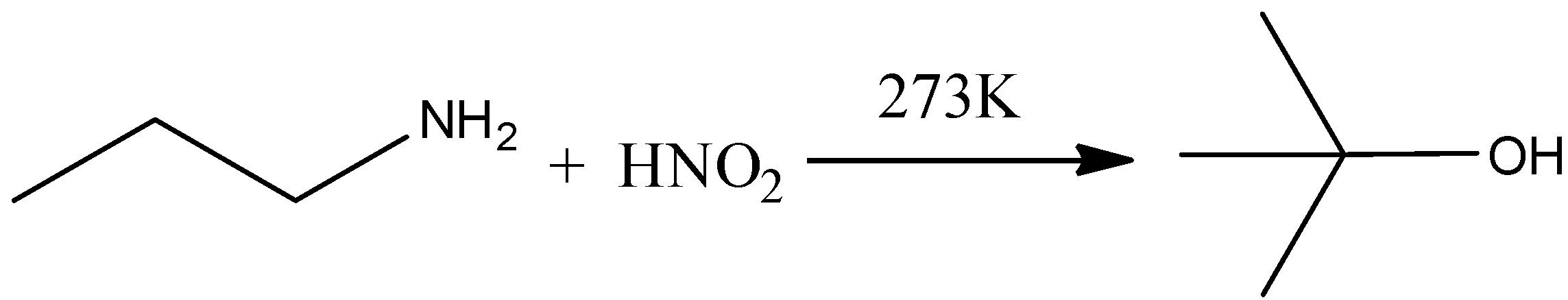



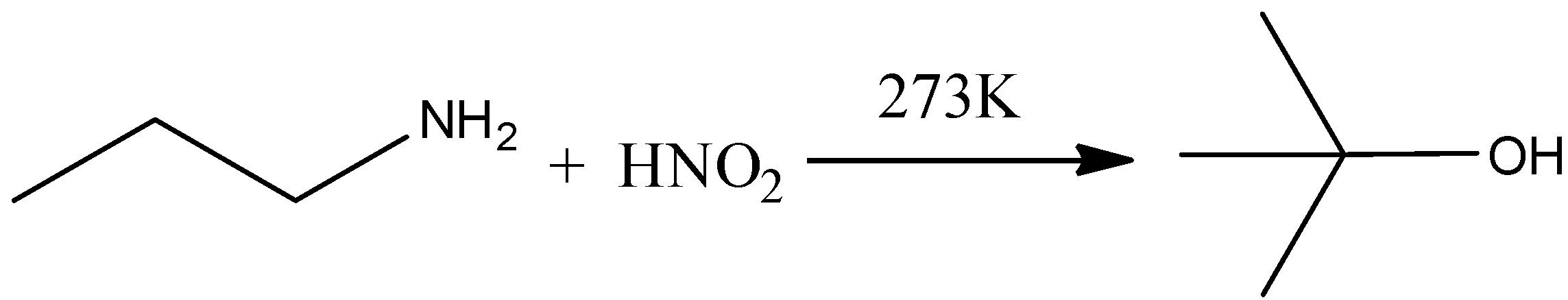
Answer
510.3k+ views
Hint: Chlorocyclohexane otherwise it is known as cyclohexyl chloride which is having the chlorinated hydrocarbon with chemical formula, \[{\left( {C{H_2}} \right)_5}CHCl\]. The second compound is \[2 - \]chloropropane is also known as isopropyl chloride with chemical formula, \[{\left( {C{H_3}} \right)_2}CHCl\]. And the last compound is a primary amine, propan\[ - 1 - \] amine otherwise it is known as n- propylamine with
chemical formula \[C{H_3}{\left( {C{H_2}} \right)_2}N{H_2}\].
Complete answer:
Here, the chlorocyclohexane is reacted with ammonia and there is a formation of cyclohexene with the elimination of ammonium chloride. Here the ammonia acts as a nucleophile. And it is the correct reaction. Hence, option (A) is incorrect.
When isopropyl chloride is reacting with ammonia, there will be a formation of isopropyl amine. Hence, the option (B) is incorrect.
When the isopropyl chloride is reacting with ammonia, there will not be a formation of isobutylene with the elimination of ammonium chloride salt. And there is only a formation of isopropyl amine. In that the halogen group is eliminated by the amino group. So, the correct reaction is,
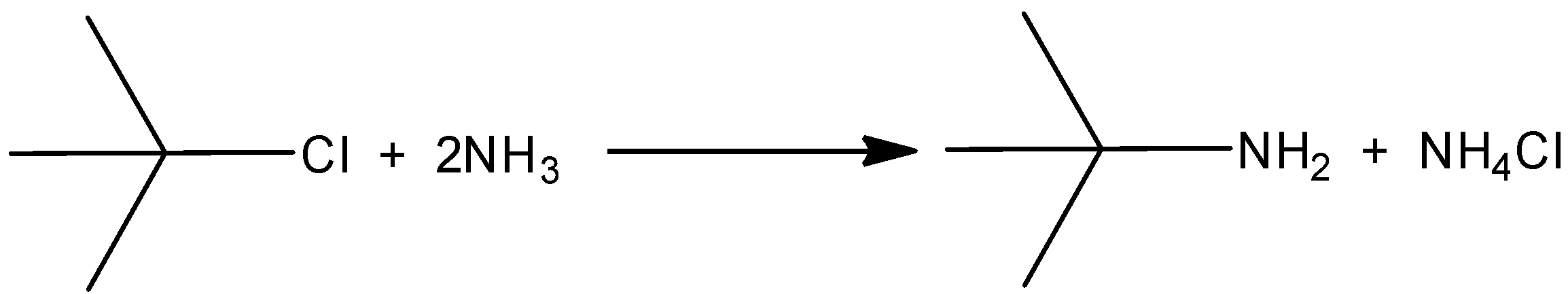
Hence, option (C) is correct.
When the propylamine is reacted with nitrous acid in the presence of heat, there is a formation of propanol. Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
We have to know that when an alkyl halide is reacting with ammonia, the reaction takes place via \[{S_N}2\] reaction. And the amine acts as the attacking nucleophile. Here the nucleophiles are uncharged. Hence, there is a positive charge present on nitrogen in the initial \[{S_N}2\] product. So, for the formation of an uncharged product, there should be an additional acid – base step.
chemical formula \[C{H_3}{\left( {C{H_2}} \right)_2}N{H_2}\].
Complete answer:
Here, the chlorocyclohexane is reacted with ammonia and there is a formation of cyclohexene with the elimination of ammonium chloride. Here the ammonia acts as a nucleophile. And it is the correct reaction. Hence, option (A) is incorrect.
When isopropyl chloride is reacting with ammonia, there will be a formation of isopropyl amine. Hence, the option (B) is incorrect.
When the isopropyl chloride is reacting with ammonia, there will not be a formation of isobutylene with the elimination of ammonium chloride salt. And there is only a formation of isopropyl amine. In that the halogen group is eliminated by the amino group. So, the correct reaction is,

Hence, option (C) is correct.
When the propylamine is reacted with nitrous acid in the presence of heat, there is a formation of propanol. Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
We have to know that when an alkyl halide is reacting with ammonia, the reaction takes place via \[{S_N}2\] reaction. And the amine acts as the attacking nucleophile. Here the nucleophiles are uncharged. Hence, there is a positive charge present on nitrogen in the initial \[{S_N}2\] product. So, for the formation of an uncharged product, there should be an additional acid – base step.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




