
Which is not a disproportionation reaction?
(A)
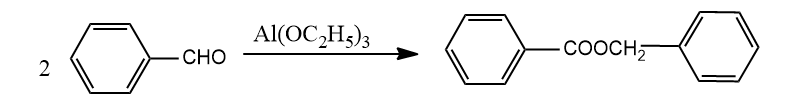
(B)
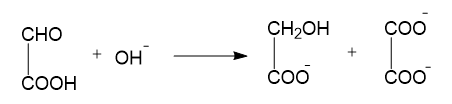
(C) $NaH+{{H}_{2}}O\to NaOH+{{H}_{2}}$
(D) All of the above


Answer
546k+ views
Hint: To answer this question, find a reaction in which the same species is both oxidized and reduced. Go through all the options and make sure you check them carefully, this question can have multiple answers also.
Complete answer:
We know that in most redox reactions something is oxidized and something else is being reduced. But in some redox reactions, one substance can be both oxidized and reduced in the same reaction and those reactions are called disproportionation reactions.
Now, we will look at all the options given to us,
In reaction A, benzaldehyde is oxidized to benzoic acid as well as reduced to benzyl alcohol. Later both these compounds react with each other to form the final compound given. So, this is a disproportionation reaction and an example of a Cannizzaro reaction.
In reaction B, oxidation, as well as reduction, takes place. Here the aldehyde group is converted to alcohol and the same aldehyde group is also converted to a carboxylic acid group. This is also an example of a Cannizzaro reaction and a disproportionation reaction.
In reaction C, hydrogen in NaH is in -1 oxidation state and in H2O is in the +1 oxidation state. The hydrogen of NaH is changing its oxidation state from -1 to +1 and Hydrogen from water changes from +1 to 0. So, this is a type of redox reaction. Hence, it is not an example of a disproportionation reaction.
Therefore, the correct answers to this question are both options A and B.
Note:
Cannizzaro reaction - We should know that the Cannizzaro reaction is a redox reaction in which two molecules of an aldehyde are reacted to produce primary alcohol and a carboxylic acid using a hydroxide base.
Complete answer:
We know that in most redox reactions something is oxidized and something else is being reduced. But in some redox reactions, one substance can be both oxidized and reduced in the same reaction and those reactions are called disproportionation reactions.
Now, we will look at all the options given to us,
In reaction A, benzaldehyde is oxidized to benzoic acid as well as reduced to benzyl alcohol. Later both these compounds react with each other to form the final compound given. So, this is a disproportionation reaction and an example of a Cannizzaro reaction.
In reaction B, oxidation, as well as reduction, takes place. Here the aldehyde group is converted to alcohol and the same aldehyde group is also converted to a carboxylic acid group. This is also an example of a Cannizzaro reaction and a disproportionation reaction.
In reaction C, hydrogen in NaH is in -1 oxidation state and in H2O is in the +1 oxidation state. The hydrogen of NaH is changing its oxidation state from -1 to +1 and Hydrogen from water changes from +1 to 0. So, this is a type of redox reaction. Hence, it is not an example of a disproportionation reaction.
Therefore, the correct answers to this question are both options A and B.
Note:
Cannizzaro reaction - We should know that the Cannizzaro reaction is a redox reaction in which two molecules of an aldehyde are reacted to produce primary alcohol and a carboxylic acid using a hydroxide base.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




