
Which is not a carbon compound?
(This question has multiple correct options)
A.Wood
B.Chalk
C.Paper
D.Cement
Answer
578.7k+ views
Hint: Carbon is the first member of group 14 elements. It forms a large number of compounds with many other elements. The most remarkable property of carbon is its catenation property because of which it can form thousands of compounds in which carbon atoms are bonded to one another.
Complete step by step answer:
The constituents of wood are water and dry matter. The dry matter, as obvious from its name, is that part of the wood which does not contain any water. The dry matter portion of the wood contains some amount of elements. It contains 50 percent of carbon, 41 percent of oxygen and 6 percent of hydrogen. Dry matter also contains other substances like nitrogen, sulphur and ash.
The flammable substances in wood are carbon and hydrogen as they burn and give out heat energy. Out of these two, carbon gives about 67 percent of heat energy while hydrogen gives out 33 percent.
Hence, wood is a carbon compound and so option A is incorrect.
Chalk is a soft, porous rock made of carbonate and is a form of limestone which is composed of the mineral calcite. It is the common name for the compound calcium carbonate. Thus, it is a calcium salt and a carbonate salt. So, option B is correct.
Paper is a thin sheet of material used for writing, for storing information, for printing, decorating and also as currency. It is chemically prepared from cellulose fibers derived from wood, rags etc. The cellulose fibers are plant fibers which contain carbon. So, paper is a carbon compound. Hence, option C is incorrect.
Cement is produced by the combination of calcium, aluminum, silicon, iron etc. It is a silicate salt. Thus, cement is not a carbon compound and so option D is correct.
Thus, options B and D are correct.
Note:
Compounds which contain only carbon and hydrogen are named as hydrocarbons. These can be further classified into saturated hydrocarbons and unsaturated hydrocarbons on the basis of the type of bonds present in them.
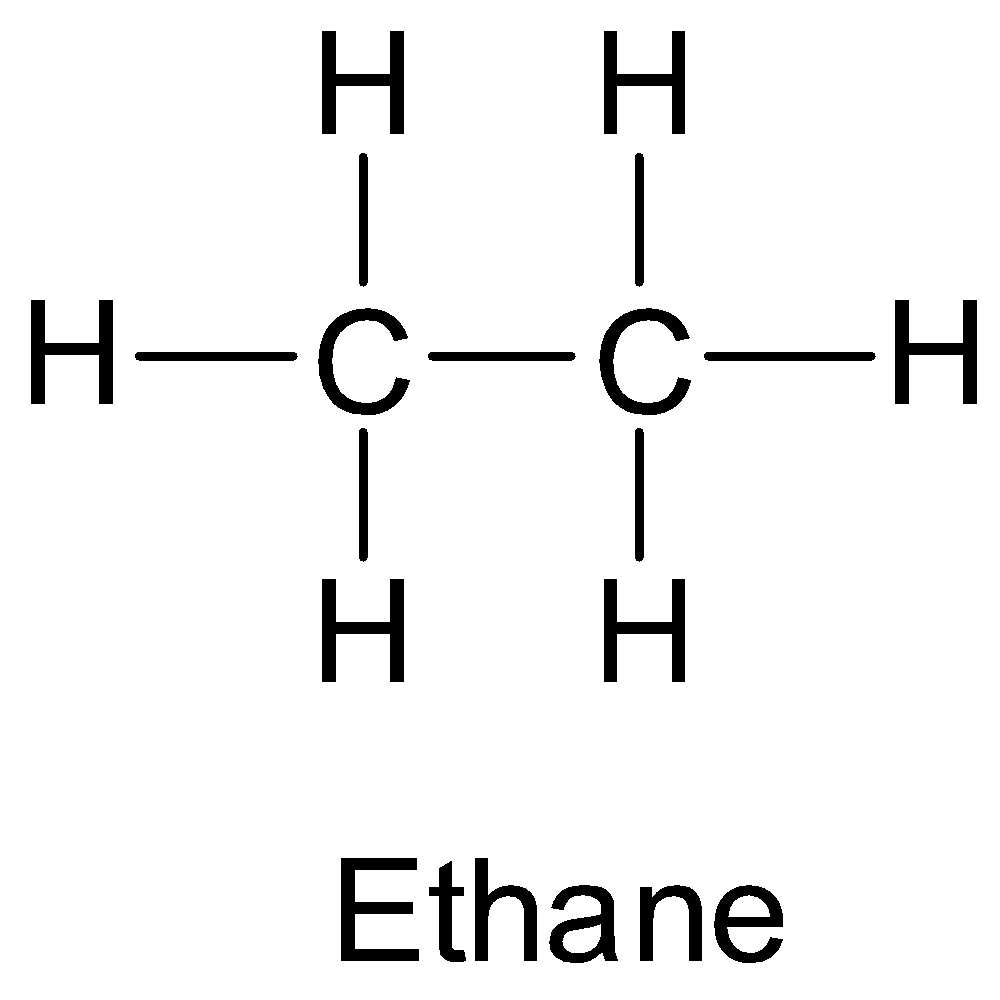
Saturated hydrocarbons have only single carbon-carbon bonds in their molecules. They are also called alkanes. For example, ethane is a saturated hydrocarbon.
Unsaturated hydrocarbons have double or triple carbon-carbon bonds in addition to single bonds. For example, ethene is an unsaturated hydrocarbon.

Complete step by step answer:
The constituents of wood are water and dry matter. The dry matter, as obvious from its name, is that part of the wood which does not contain any water. The dry matter portion of the wood contains some amount of elements. It contains 50 percent of carbon, 41 percent of oxygen and 6 percent of hydrogen. Dry matter also contains other substances like nitrogen, sulphur and ash.
The flammable substances in wood are carbon and hydrogen as they burn and give out heat energy. Out of these two, carbon gives about 67 percent of heat energy while hydrogen gives out 33 percent.
Hence, wood is a carbon compound and so option A is incorrect.
Chalk is a soft, porous rock made of carbonate and is a form of limestone which is composed of the mineral calcite. It is the common name for the compound calcium carbonate. Thus, it is a calcium salt and a carbonate salt. So, option B is correct.
Paper is a thin sheet of material used for writing, for storing information, for printing, decorating and also as currency. It is chemically prepared from cellulose fibers derived from wood, rags etc. The cellulose fibers are plant fibers which contain carbon. So, paper is a carbon compound. Hence, option C is incorrect.
Cement is produced by the combination of calcium, aluminum, silicon, iron etc. It is a silicate salt. Thus, cement is not a carbon compound and so option D is correct.
Thus, options B and D are correct.
Note:
Compounds which contain only carbon and hydrogen are named as hydrocarbons. These can be further classified into saturated hydrocarbons and unsaturated hydrocarbons on the basis of the type of bonds present in them.
Saturated hydrocarbons have only single carbon-carbon bonds in their molecules. They are also called alkanes. For example, ethane is a saturated hydrocarbon.

Unsaturated hydrocarbons have double or triple carbon-carbon bonds in addition to single bonds. For example, ethene is an unsaturated hydrocarbon.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




