
Which is more stable, cis -$1$-ethyl-$2$ -methylcyclohexane or trans -$1$-ethyl-$2$-methylcyclohexane?
Answer
478.2k+ views
Hint: There is unfastened rotation approximately the carbon-to-carbon unmarried bonds (C–C) in alkanes. In contrast, the shape of alkenes calls for that the carbon atoms of a double bond and the $2$ atoms bonded to every carbon atom all lie in an unmarried plane, and that every doubly bonded carbon atom lies withinside the middle of a triangle. This part of the molecule’s shape is rigid; rotation of approximately doubly bonded carbon atoms isn't feasible without rupturing the bond.
Complete Answer:
Trans -$1$-ethyl-$2$-methylcyclohexane will be more stable than cis-$1$-ethyl-$2$-methylcyclohexane
I'll examine the $2$maximum solid chair conformations for every molecule. If you need to look at why those chairs are the maximum solid, see
Now, this is the maximum solid chair conformer for trans-$1$-ethyl-$2$-methylcyclohexane
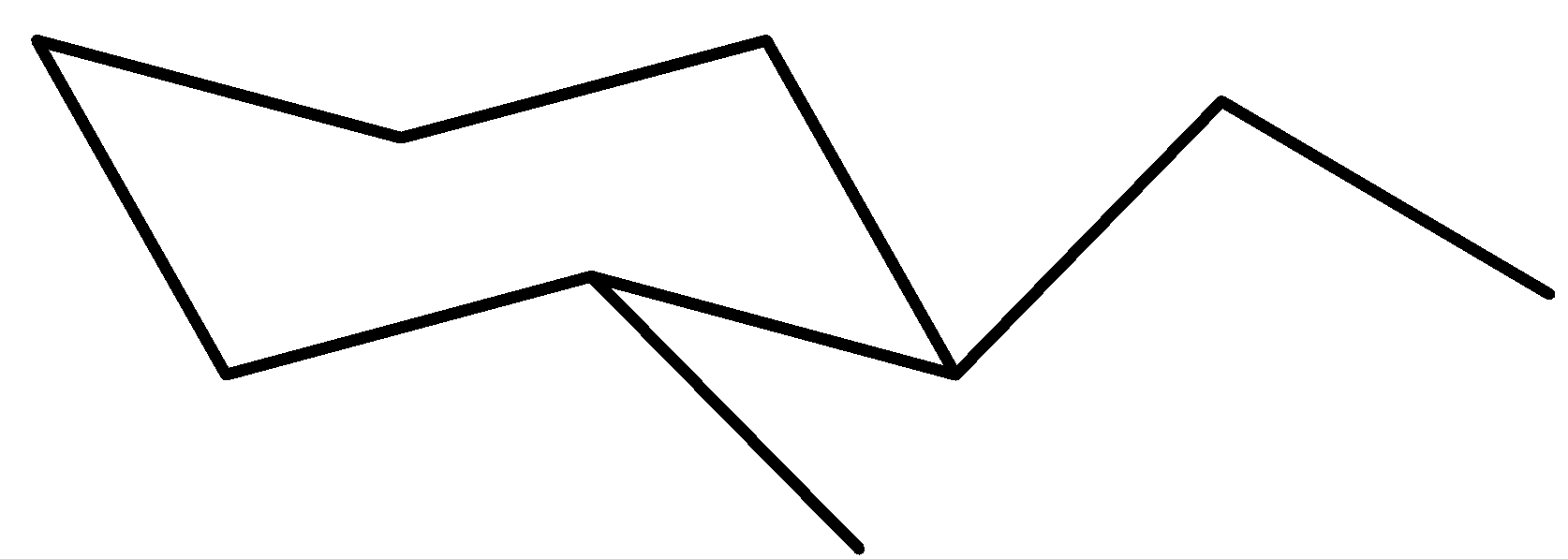
Notice which you have the ethyl organization connected to carbon ($1$) in UP function on an equatorial bond. The methyl organization is hooked up to carbon ($2$) in DOWN function on an equatorial bond as well.
Now examine the maximum solid chair for cis-$1$-ethyl-$2$-methylcyclohexane
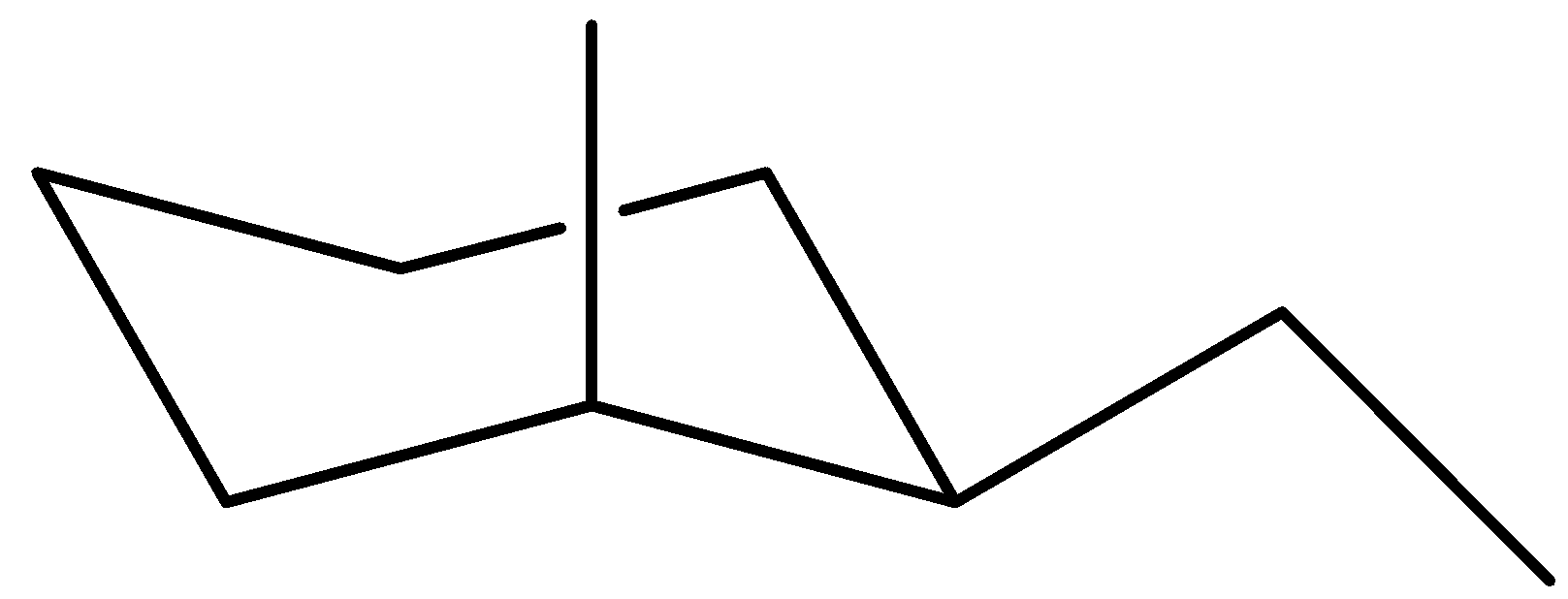
Once again, the ethyl organization is in UP function on carbon ($1$), however this time the methyl organization is in UP function on an axial bond. The reality that the methyl organization is on an axial bond will in the end decide which of those chair conformers are extra solid.
Ideally, extra solid chairs will have the bigger companies on equatorial bonds. As you may see, that's what takes place withinside the first chair. For the second chair, the methyl organization's function on an axial bond will purpose steric strain, as a way to lessen the steadiness of the chair.
As a result, trans-$1$-ethyl-$2$-methylcyclohexane has an extra solid chair conformer than cis-$1$-ethyl-$2$-methylcyclohexane.
Note:
If you may choose up both molecule from the web page and turn it over from pinnacle to bottom, you'll see that the $2$ formulation are identical. Thus there are necessities for cis-trans isomerism:
$1$. Rotation needs to be restrained withinside the molecule.
$2$. There need to be no identical companies on every doubly bonded carbon atom.
Complete Answer:
Trans -$1$-ethyl-$2$-methylcyclohexane will be more stable than cis-$1$-ethyl-$2$-methylcyclohexane
I'll examine the $2$maximum solid chair conformations for every molecule. If you need to look at why those chairs are the maximum solid, see
Now, this is the maximum solid chair conformer for trans-$1$-ethyl-$2$-methylcyclohexane

Notice which you have the ethyl organization connected to carbon ($1$) in UP function on an equatorial bond. The methyl organization is hooked up to carbon ($2$) in DOWN function on an equatorial bond as well.
Now examine the maximum solid chair for cis-$1$-ethyl-$2$-methylcyclohexane

Once again, the ethyl organization is in UP function on carbon ($1$), however this time the methyl organization is in UP function on an axial bond. The reality that the methyl organization is on an axial bond will in the end decide which of those chair conformers are extra solid.
Ideally, extra solid chairs will have the bigger companies on equatorial bonds. As you may see, that's what takes place withinside the first chair. For the second chair, the methyl organization's function on an axial bond will purpose steric strain, as a way to lessen the steadiness of the chair.
As a result, trans-$1$-ethyl-$2$-methylcyclohexane has an extra solid chair conformer than cis-$1$-ethyl-$2$-methylcyclohexane.
Note:
If you may choose up both molecule from the web page and turn it over from pinnacle to bottom, you'll see that the $2$ formulation are identical. Thus there are necessities for cis-trans isomerism:
$1$. Rotation needs to be restrained withinside the molecule.
$2$. There need to be no identical companies on every doubly bonded carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




