
Which is more Acidic Toluene or Phenol?
Answer
508.5k+ views
Hint: Acidic molecules contain structural characteristics that allow the conjugate base anion to delocalize its charge over a broader area. The molecule is more stable when the negative charge is delocalized (so that one atom does not have to bear the entire negative charge).
Complete answer:
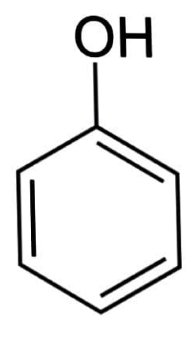
Let us know about phenol. The aromatic organic chemical phenol (also known as carbolic acid) has the chemical formula \[{C_6}{H_5}OH\]. It is a flammable white crystalline substance. A phenyl group \[\left( {{C_6}{H_5}} \right)\] is connected to a hydroxyl group \[\left( {OH} \right)\] in this molecule. It's mildly acidic and should be handled with caution because it can cause chemical burns.
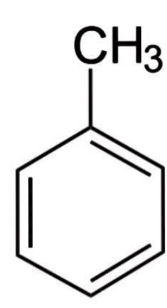
Now let us get some ideas about Toluene. Toluene is an aromatic hydrocarbon that is also known as toluol. It's a colourless, water-insoluble liquid that has a paint thinner-like odour. It's a phenyl group connected to a methyl group (\[C{H_3}\]) in a mono-substituted benzene derivative. As a result, its IUPAC designation is methylbenzene. Toluene is primarily utilised as a solvent and an industrial feedstock.
Because phenol has an \[OH\] group, it is more acidic than toluene.
Note:
Phenol is a widely used chemical that can be found in a variety of products, including air fresheners, aftershave, bronchial mists, chloraseptic throat spray, deodorants, feminine powders and sprays, hair spray, decongestants, mouthwash, aspirin, solvents, acne medications, antiseptics, calamine lotions, and cleaning products.
Toluene has a wide range of commercial and industrial uses, including as a solvent in paints, lacquers, thinners, glues, correction fluid, and nail polish remover, as well as in the printing and tanning processes.
Complete answer:
Let us know about phenol. The aromatic organic chemical phenol (also known as carbolic acid) has the chemical formula \[{C_6}{H_5}OH\]. It is a flammable white crystalline substance. A phenyl group \[\left( {{C_6}{H_5}} \right)\] is connected to a hydroxyl group \[\left( {OH} \right)\] in this molecule. It's mildly acidic and should be handled with caution because it can cause chemical burns.

Now let us get some ideas about Toluene. Toluene is an aromatic hydrocarbon that is also known as toluol. It's a colourless, water-insoluble liquid that has a paint thinner-like odour. It's a phenyl group connected to a methyl group (\[C{H_3}\]) in a mono-substituted benzene derivative. As a result, its IUPAC designation is methylbenzene. Toluene is primarily utilised as a solvent and an industrial feedstock.

Because phenol has an \[OH\] group, it is more acidic than toluene.
Note:
Phenol is a widely used chemical that can be found in a variety of products, including air fresheners, aftershave, bronchial mists, chloraseptic throat spray, deodorants, feminine powders and sprays, hair spray, decongestants, mouthwash, aspirin, solvents, acne medications, antiseptics, calamine lotions, and cleaning products.
Toluene has a wide range of commercial and industrial uses, including as a solvent in paints, lacquers, thinners, glues, correction fluid, and nail polish remover, as well as in the printing and tanning processes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




