
Which is an incorrect statement.
(A) The given structures are conformations.
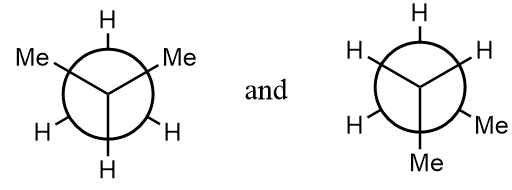
(B) The given structure is a meso compound.
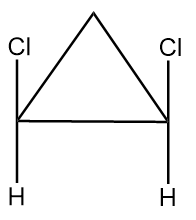
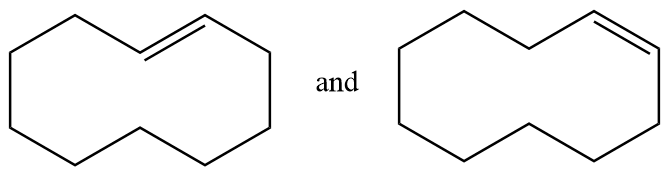
(C) The given structures are geometrical isomers.
(D) The given structures are enantiomers.
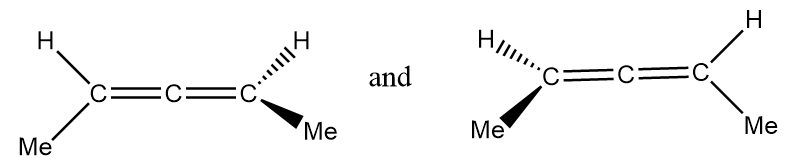
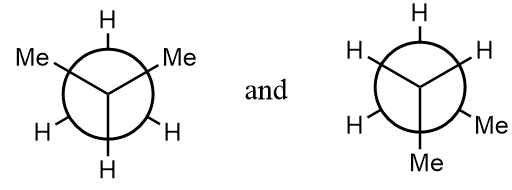


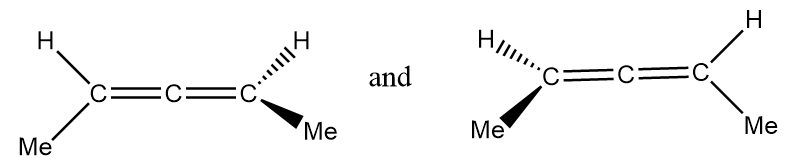
Answer
513.9k+ views
Hint : When more than one compound consists of the same molecular formula but differs in the arrangement of atoms in space are known as stereoisomers and the phenomenon is known as stereoisomerism. It is broadly divided into two parts i.e., optical isomerism and geometrical isomerism.
Complete Step By Step Answer:
Let us have a look at each given pair of compounds separately.
Pair of compounds given in option-(A) are as follows:
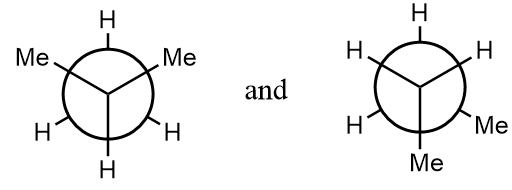
The normal structures of the given compounds are as follows:
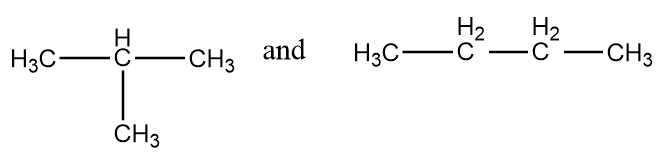
As it is clearly observed that the first structure is isobutane and the second structure is n-butane i.e., the structures are not same and hence can not form stereoisomers. The given structures are constitutional isomers of each other. Hence, it is an incorrect statement.
Compound given in option (B) is as follows:
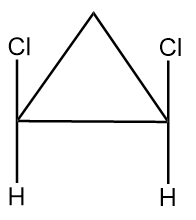
As it is clearly observed that the compound contains two chiral carbon atoms and there also exist a plane of symmetry in the compound as shown below:
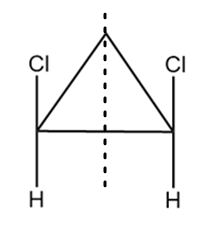
Hence, the given compound is a meso compound.
Pair of structures given in option-(C) are as follows:
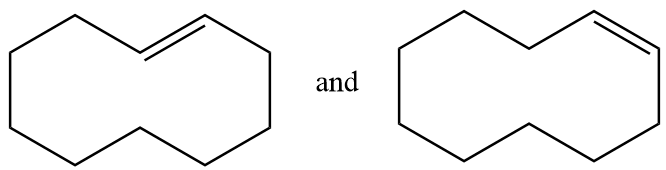
The hydrogen atom near the double bonds of the ring can be aligned as follows:
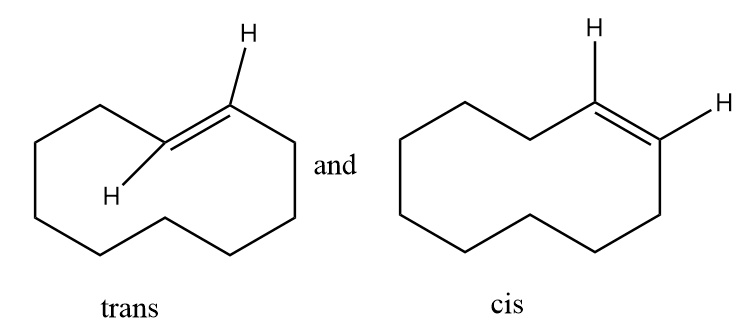
In structure-1, the hydrogen atoms are aligned to the opposite side of double bond so it is representing trans isomer whereas in structure-2, the hydrogen atoms are aligned to the same side of the double bond so it represents cis isomer. Hence the given pair of structures are geometrical isomers of each other.
Pair of structures given in option-(D) are as follows:
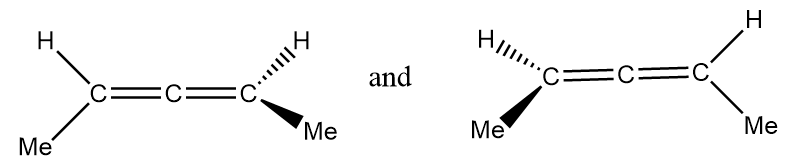
It is clearly observed that the given structures are non-superimposable mirror images of each other so the given structures represent a pair of enantiomers.
Hence, option (A) is the correct answer.
Note :
It is important to note that for a compound to be meso, it must have at least two chiral carbons and it must possess a plane of symmetry or centre of symmetry. Also, the stereoisomers only formed when arrangement of groups differ in space but if the position of a group is changing or there is a change in bonding, then it will not be considered as stereoisomerism.
Complete Step By Step Answer:
Let us have a look at each given pair of compounds separately.
Pair of compounds given in option-(A) are as follows:
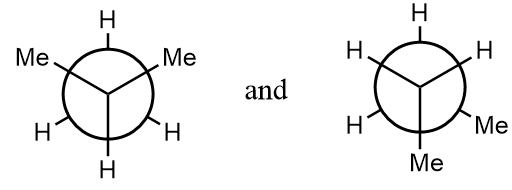
The normal structures of the given compounds are as follows:
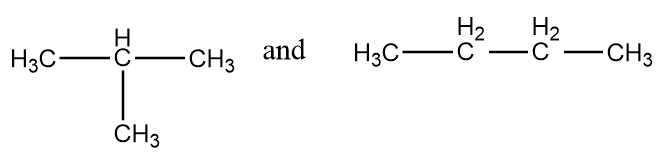
As it is clearly observed that the first structure is isobutane and the second structure is n-butane i.e., the structures are not same and hence can not form stereoisomers. The given structures are constitutional isomers of each other. Hence, it is an incorrect statement.
Compound given in option (B) is as follows:

As it is clearly observed that the compound contains two chiral carbon atoms and there also exist a plane of symmetry in the compound as shown below:

Hence, the given compound is a meso compound.
Pair of structures given in option-(C) are as follows:

The hydrogen atom near the double bonds of the ring can be aligned as follows:

In structure-1, the hydrogen atoms are aligned to the opposite side of double bond so it is representing trans isomer whereas in structure-2, the hydrogen atoms are aligned to the same side of the double bond so it represents cis isomer. Hence the given pair of structures are geometrical isomers of each other.
Pair of structures given in option-(D) are as follows:
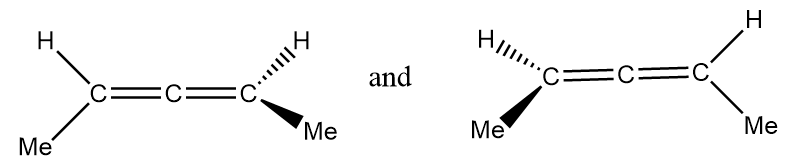
It is clearly observed that the given structures are non-superimposable mirror images of each other so the given structures represent a pair of enantiomers.
Hence, option (A) is the correct answer.
Note :
It is important to note that for a compound to be meso, it must have at least two chiral carbons and it must possess a plane of symmetry or centre of symmetry. Also, the stereoisomers only formed when arrangement of groups differ in space but if the position of a group is changing or there is a change in bonding, then it will not be considered as stereoisomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




