
Which has an electronic configuration
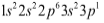
(A)
(B) Al
(C) F
(D) Ti
(E) B
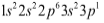
Answer
564.3k+ views
Hint: The given electronic configuration belongs to the p block as the last electron enters in the p orbital. They are representative elements. The elements of group one are called p block elements. We will find the period and the group number and accordingly find the element associated with this configuration.
Complete answer: We know that the p block elements occupying the left part of the periodic table. Here it tells us that it is present in the third row of the periodic table. The ‘p ‘ here tells us that it is present in the p block which is right to the transition metals. The superscript tells us that it is present in the first column of the p block group thirteen. On simplifying it we get that this element has thirteen electrons which is equal to the number of protons which is equal to the atomic number Z. So the element with atomic number is aluminium. It is present in the th group and rd period and it is a part of the boron family.
So the given electronic configuration of the element is of aluminium.
Hence the correct answer is option (B).
Note: This electronic configuration is valid with the assumption that aluminium does not lose or gain an electron to convert to ion. As the atomic number is determined by the protons and not the electrons. This holds for we have assumed it as a neutral atom of an element. So this should be kept in mind.
Complete answer: We know that the p block elements occupying the left part of the periodic table. Here it tells us that it is present in the third row of the periodic table. The ‘p ‘ here tells us that it is present in the p block which is right to the transition metals. The superscript tells us that it is present in the first column of the p block group thirteen. On simplifying it we get that this element has thirteen electrons which is equal to the number of protons which is equal to the atomic number Z. So the element with atomic number is aluminium. It is present in the th group and rd period and it is a part of the boron family.
So the given electronic configuration of the element is of aluminium.
Hence the correct answer is option (B).
Note: This electronic configuration is valid with the assumption that aluminium does not lose or gain an electron to convert to ion. As the atomic number is determined by the protons and not the electrons. This holds for we have assumed it as a neutral atom of an element. So this should be kept in mind.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




