
Which has a linear structure?
(This question has multiple correct option)
A. $Be{{F}_{2}}$
B. $Ag{{[CN]}_{2}}$
C. $C{{O}_{2}}$
D. $Xe{{F}_{2}}$
Answer
541.8k+ views
Hint: To solve this question we have to find the hybridization of each of them. To find the hybridization of each central atom we have to calculate the lone pairs and the bond pairs of the atom and if hydrocarbon then the total sigma bonds. Here we have to find the molecule with linear structure and the linear structure will have 'sp' hybridization and the angle between the atoms will be ${{180}^{o}}$ . Or we can say that the arrangement of lone pair and bond pair will be in such a manner that the angle between the bond pairs will be ${{180}^{o}}$ and thus the structure will be linear. We can also use VSEPR theory to find the answer.
Complete answer:
From your chemistry lesson you have learned about the structures of molecules and their hybridization. Here we are going to find the hybridization by finding the lone pair and the bond pair and thus determine the bond angles and then we will draw the structures of each of them and find the answer.
A. $Be{{F}_{2}}$
As we know that beryllium has 2 electrons in their valence shell and fluorine has 7 electrons in their valence shell therefore the beryllium shares 1 electron with each of the fluorine atoms and thus forms the two sigma bonds.
Therefore, hybrid orbitals = no. of sigma bonds = 2.
So, the hybridization will be 'sp' and hence the structure will be linear.
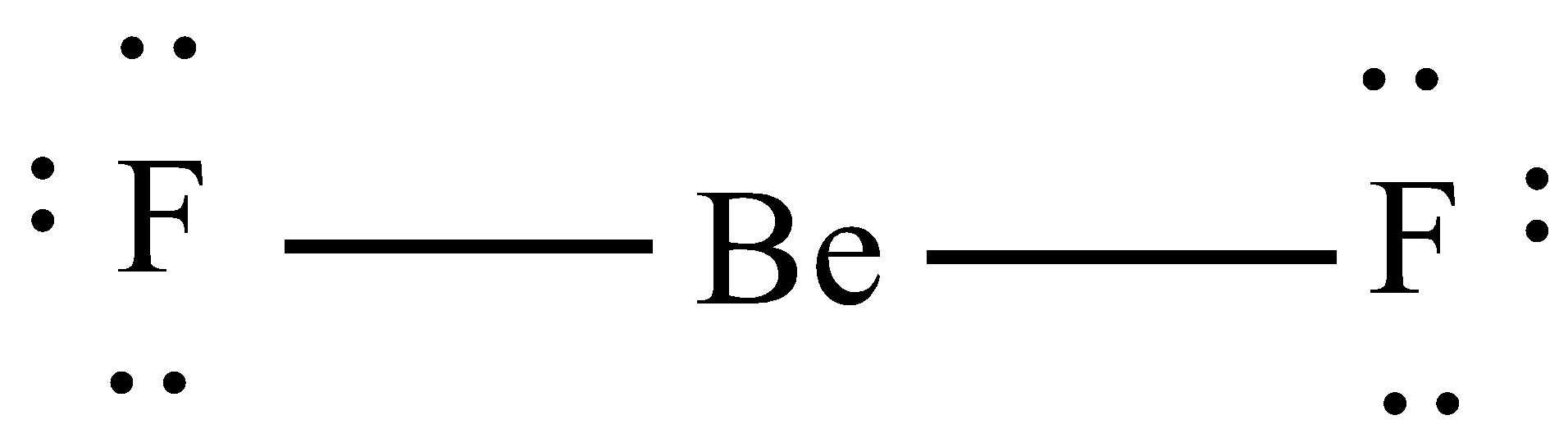
B. $Ag{{[CN]}_{2}}$
Here the central atom will be Ag (silver) as the valence electron in Ag is one only and thus it carries a positive charge and the cyanide ion has a uninegative charge on it and thus one unipositive and one uninegative charge is balanced and the lone pair present on the C in the monodentate CN ligand tends to form the coordinate bond with the Ag atom. And hence, have the hybridization of 'sp' and thus the structure will be linear.
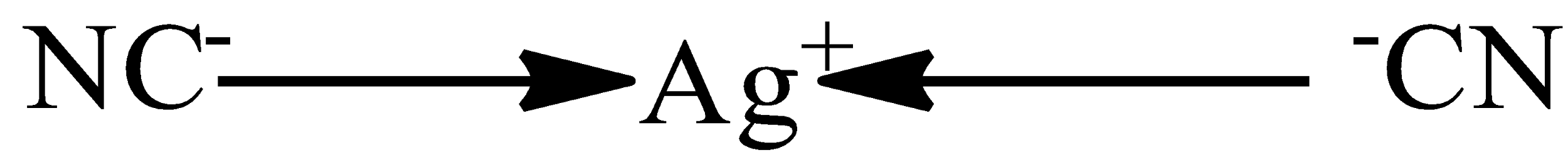
C. $C{{O}_{2}}$
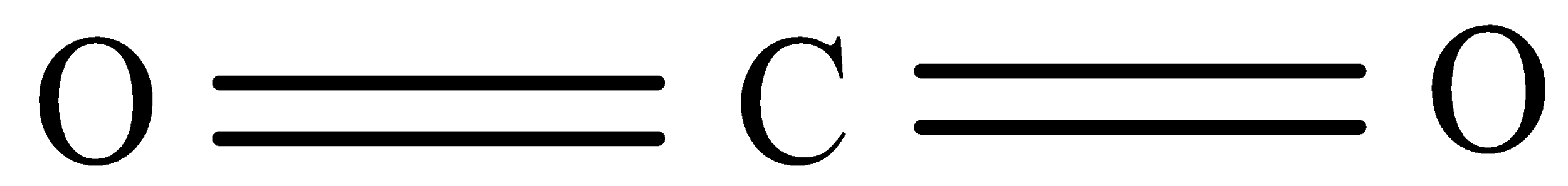
Here the central atom will be Carbon which has four valence electrons in their valence shell and oxygen will have six electrons in their valence shell therefore the carbon shares 2 electrons with each of the oxygen atom and thus form 2 sigma and 2 pi bonds.
Hybrid orbitals = no. of sigma bonds = 2
So, the hybridization will be 'sp' and thus the structure will be linear.
D. $Xe{{F}_{2}}$
Here the central atom will be xenon (Xe) which has 8 electrons in their valence shell and fluorine will have 7 electrons in their valence shell therefore xenon will share 1 electron with each of the fluorine and thus form 2 sigma bonds.
Hybrid orbitals = no. of sigma bonds = 2
So, the hybridization will be 'sp' and thus the structure will be linear.
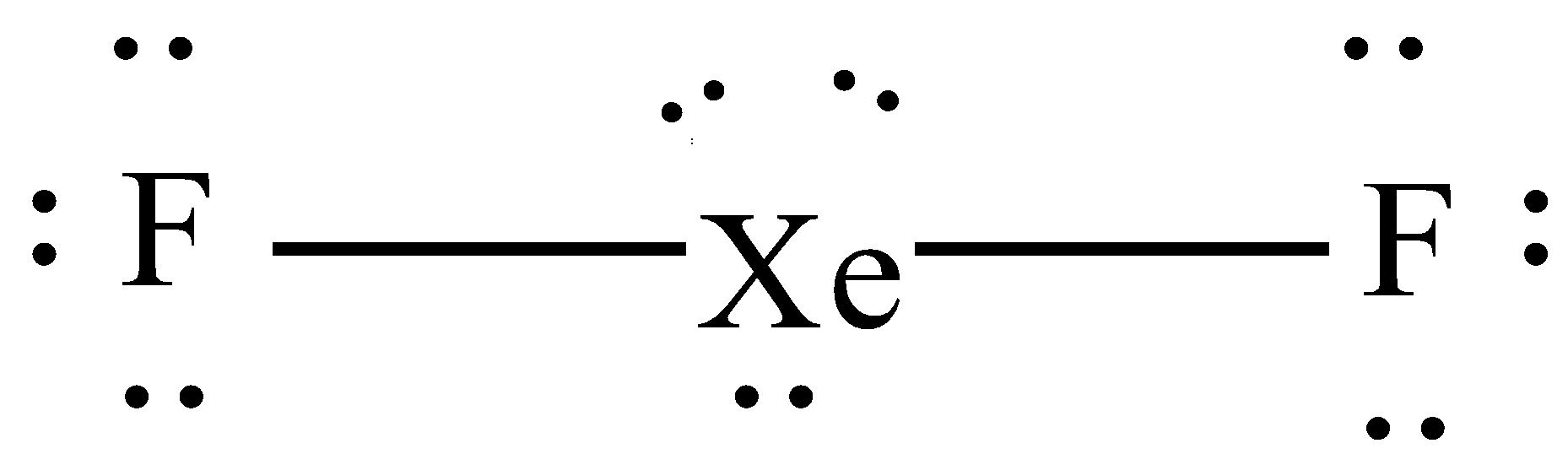
Therefore all the molecules will have linear structure.
Thus the correct option will be (A), (B), (C) and (D).
Note:
Hybridization of the molecules can also be calculated by other ways. There is one shortcut alternative to find the hybridization of the molecule with one central atom which is given as
Hybrid orbitals = $\dfrac{1}{2}$[valence electrons of central atom + monovalent atoms - magnitude of positive charge on central atom + magnitude of negative charge on central atom].
Complete answer:
From your chemistry lesson you have learned about the structures of molecules and their hybridization. Here we are going to find the hybridization by finding the lone pair and the bond pair and thus determine the bond angles and then we will draw the structures of each of them and find the answer.
A. $Be{{F}_{2}}$
As we know that beryllium has 2 electrons in their valence shell and fluorine has 7 electrons in their valence shell therefore the beryllium shares 1 electron with each of the fluorine atoms and thus forms the two sigma bonds.
Therefore, hybrid orbitals = no. of sigma bonds = 2.
So, the hybridization will be 'sp' and hence the structure will be linear.
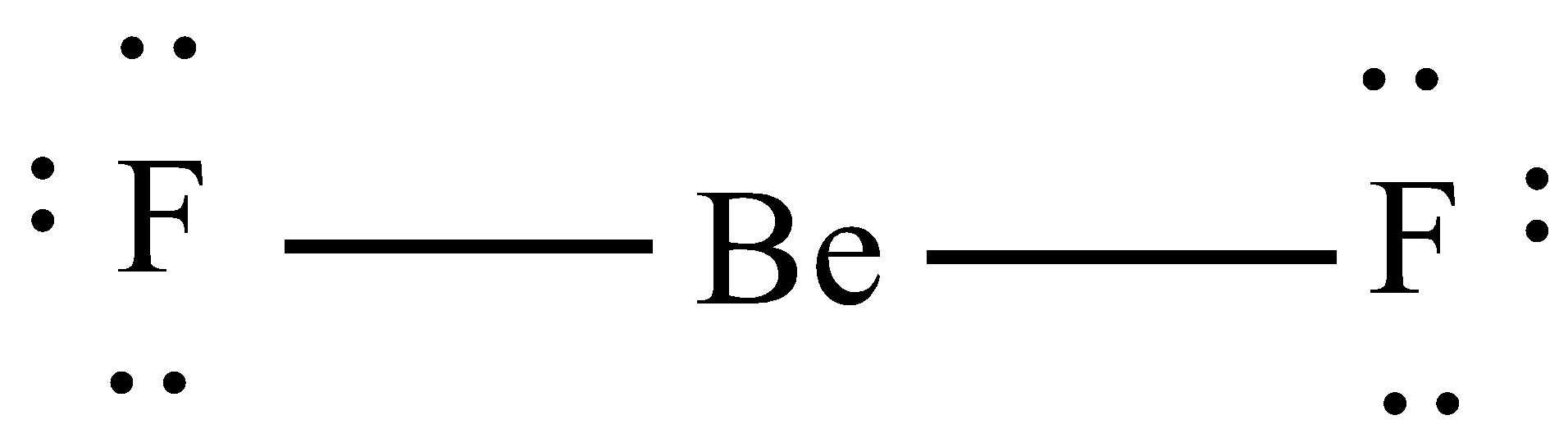
B. $Ag{{[CN]}_{2}}$
Here the central atom will be Ag (silver) as the valence electron in Ag is one only and thus it carries a positive charge and the cyanide ion has a uninegative charge on it and thus one unipositive and one uninegative charge is balanced and the lone pair present on the C in the monodentate CN ligand tends to form the coordinate bond with the Ag atom. And hence, have the hybridization of 'sp' and thus the structure will be linear.
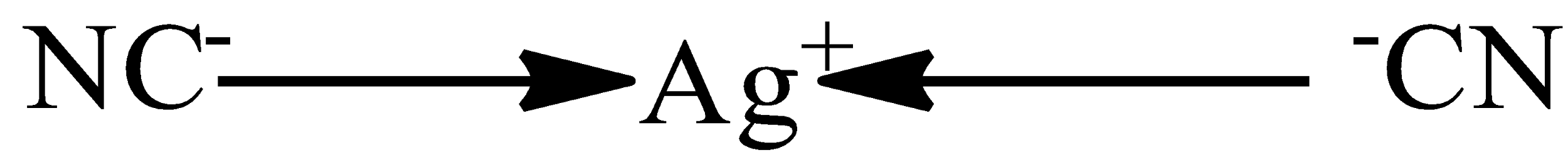
C. $C{{O}_{2}}$
Here the central atom will be Carbon which has four valence electrons in their valence shell and oxygen will have six electrons in their valence shell therefore the carbon shares 2 electrons with each of the oxygen atom and thus form 2 sigma and 2 pi bonds.
Hybrid orbitals = no. of sigma bonds = 2
So, the hybridization will be 'sp' and thus the structure will be linear.

D. $Xe{{F}_{2}}$
Here the central atom will be xenon (Xe) which has 8 electrons in their valence shell and fluorine will have 7 electrons in their valence shell therefore xenon will share 1 electron with each of the fluorine and thus form 2 sigma bonds.
Hybrid orbitals = no. of sigma bonds = 2
So, the hybridization will be 'sp' and thus the structure will be linear.
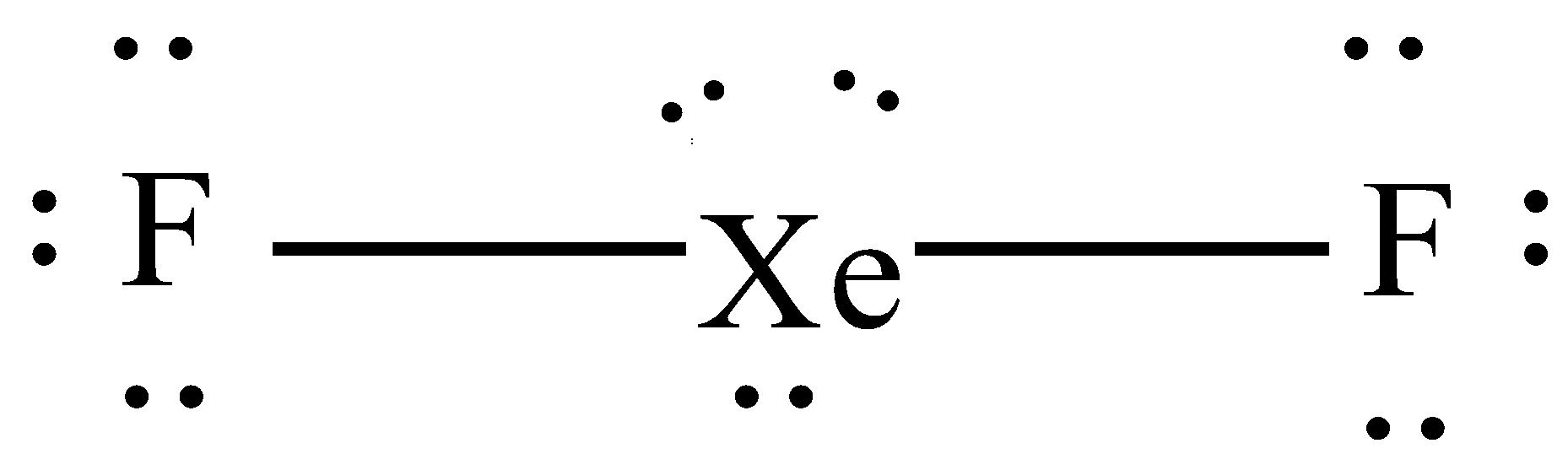
Therefore all the molecules will have linear structure.
Thus the correct option will be (A), (B), (C) and (D).
Note:
Hybridization of the molecules can also be calculated by other ways. There is one shortcut alternative to find the hybridization of the molecule with one central atom which is given as
Hybrid orbitals = $\dfrac{1}{2}$[valence electrons of central atom + monovalent atoms - magnitude of positive charge on central atom + magnitude of negative charge on central atom].
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




