
Which gas is the antidote of Lewisite (a poisonous gas used during World War II)?
[A] Sarin gas
[B] MIC
[C] BAL
[D] Mustard gas
Answer
592.8k+ views
Hint: Lewisite is a poisonous compound containing arsenic and its liquid form is stronger than the gaseous form. Its antidote has a property of forming chelates with the poisonous metal which is excreted through urine.
Complete step by step answer:
Lewisite is an organic compound containing arsenic. It was used during World War II by the Soviet Union, Japan, Germany, The U.S and Britain.
It was used as a chemical warfare agent and it was used as a blistering agent as it caused chemical burns which resulted in formation of water like blisters on the affected bodies. It caused skin and eye irritation and also lung irritation.
Lewisite is oily and colourless in its pure form but appears brownish-black in its impure form.
It was prepared by adding arsenic trichloride to acetylene in presence of a catalyst. We can write the reaction as-
\[AsC{{l}_{3}}+{{C}_{2}}{{H}_{2}}\to ClCHCHAsC{{l}_{2}}\]
Arsenic trichloride + acetylene $\to$ 2-chlorovinylarsonous dichloride (Lewisite)
Lewisite was first synthesised in 1904.
Now we will discuss about the effect of this poisonous gas:
- It easily penetrates latex gloves and ordinary clothes and coming in contact with the skin immediately causes a burning pain and itching. Fluid-filled blisters are formed within 12 hours and sufficient absorption may even cause destruction of cells in the liver.
- It causes damage to the capillaries which causes insufficient blood flow for maintenance of optimal blood pressure hence causes low blood pressure which may result in kidney damage.
- Inhaling the gas might cause nose bleed, severe coughing, vomiting and difficulty in breathing.
- Long exposure to Lewisite might cause arsenic poisoning and permanent damage to several organs and eye exposure might cause permanent loss of sight.
However, a British medication named Dimercaprol is the antidote of Lewisite which can be used for treating arsenic, lead, mercury and gold poisoning and is given as an injection in the muscle.
Dimercaprol is a dithiol and its formula is${{C}_{3}}{{H}_{8}}O{{S}_{2}}$. It forms a chelate with the poisonous metal ion and is excreted through urine.
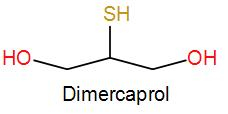
As it was a British antidote to Lewisite, it was abbreviated as BAL.
Therefore, the correct answer is option [C] BAL.
Note: Arsenic affects the metabolic enzymes by chemically reacting with adjacent thiols that affects the enzymes activity. Dimercaprol is a thiol itself and it competes with the other thiols to form a chelate with the poisonous metal. Dimercaprol is itself toxic and has many side effects like kidney poisoning and high blood pressure.
Complete step by step answer:
Lewisite is an organic compound containing arsenic. It was used during World War II by the Soviet Union, Japan, Germany, The U.S and Britain.
It was used as a chemical warfare agent and it was used as a blistering agent as it caused chemical burns which resulted in formation of water like blisters on the affected bodies. It caused skin and eye irritation and also lung irritation.
Lewisite is oily and colourless in its pure form but appears brownish-black in its impure form.
It was prepared by adding arsenic trichloride to acetylene in presence of a catalyst. We can write the reaction as-
\[AsC{{l}_{3}}+{{C}_{2}}{{H}_{2}}\to ClCHCHAsC{{l}_{2}}\]
Arsenic trichloride + acetylene $\to$ 2-chlorovinylarsonous dichloride (Lewisite)
Lewisite was first synthesised in 1904.
Now we will discuss about the effect of this poisonous gas:
- It easily penetrates latex gloves and ordinary clothes and coming in contact with the skin immediately causes a burning pain and itching. Fluid-filled blisters are formed within 12 hours and sufficient absorption may even cause destruction of cells in the liver.
- It causes damage to the capillaries which causes insufficient blood flow for maintenance of optimal blood pressure hence causes low blood pressure which may result in kidney damage.
- Inhaling the gas might cause nose bleed, severe coughing, vomiting and difficulty in breathing.
- Long exposure to Lewisite might cause arsenic poisoning and permanent damage to several organs and eye exposure might cause permanent loss of sight.
However, a British medication named Dimercaprol is the antidote of Lewisite which can be used for treating arsenic, lead, mercury and gold poisoning and is given as an injection in the muscle.
Dimercaprol is a dithiol and its formula is${{C}_{3}}{{H}_{8}}O{{S}_{2}}$. It forms a chelate with the poisonous metal ion and is excreted through urine.

As it was a British antidote to Lewisite, it was abbreviated as BAL.
Therefore, the correct answer is option [C] BAL.
Note: Arsenic affects the metabolic enzymes by chemically reacting with adjacent thiols that affects the enzymes activity. Dimercaprol is a thiol itself and it competes with the other thiols to form a chelate with the poisonous metal. Dimercaprol is itself toxic and has many side effects like kidney poisoning and high blood pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




