
Which formula correctly represents the bonding capacity of the atom involved?
This question has multiple answers.
A)
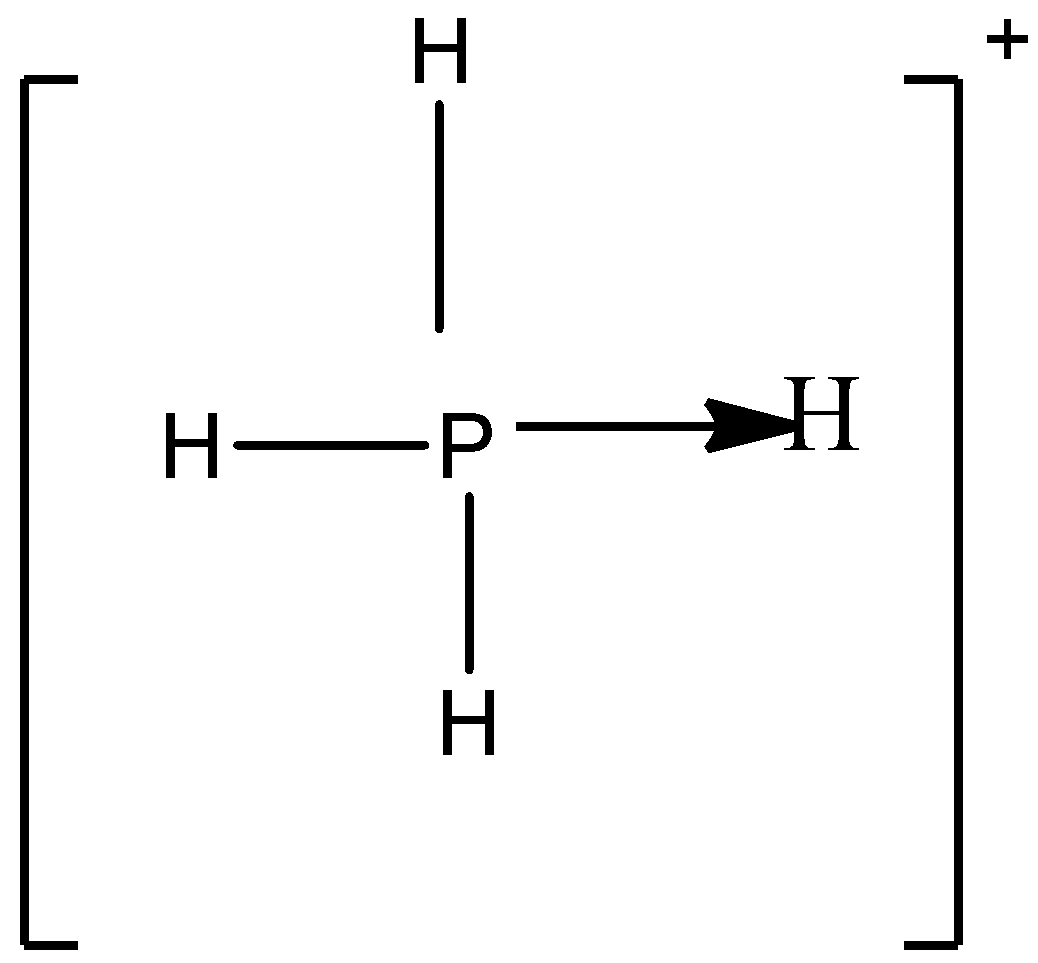
B)
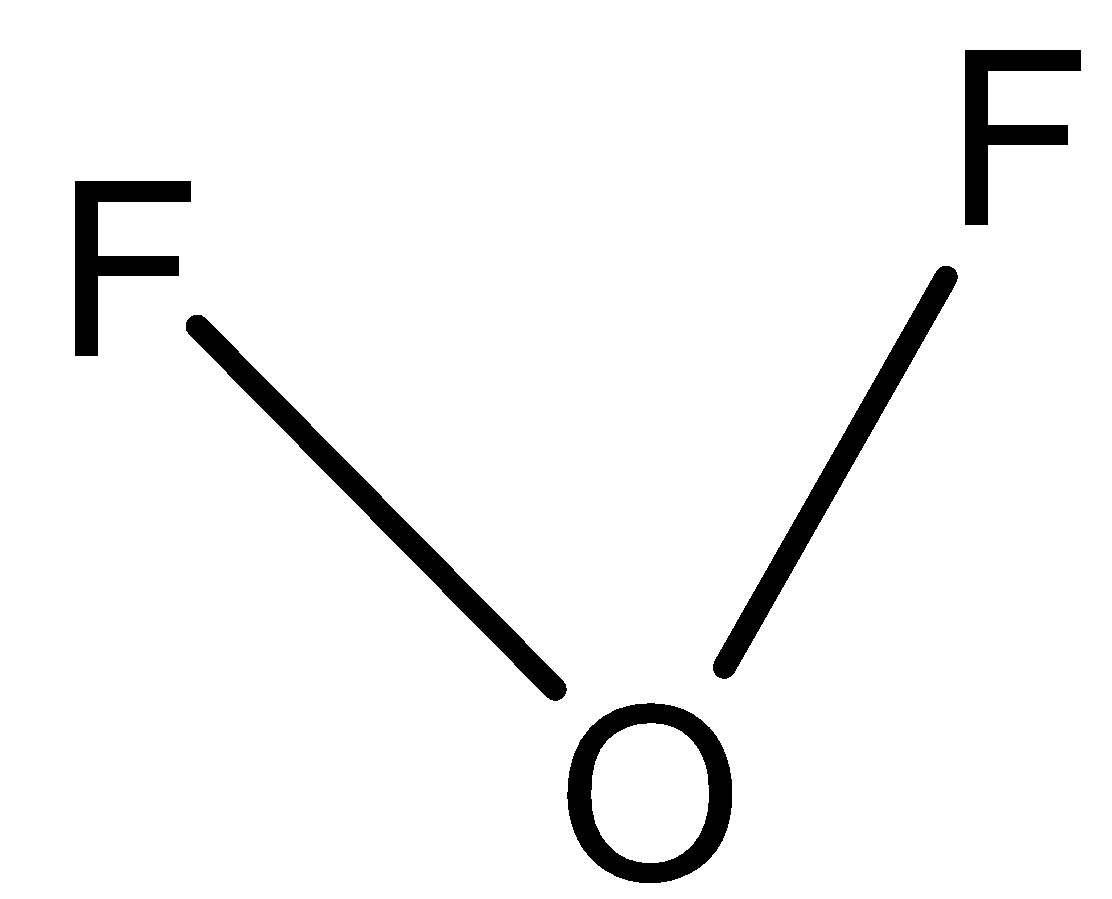
C)
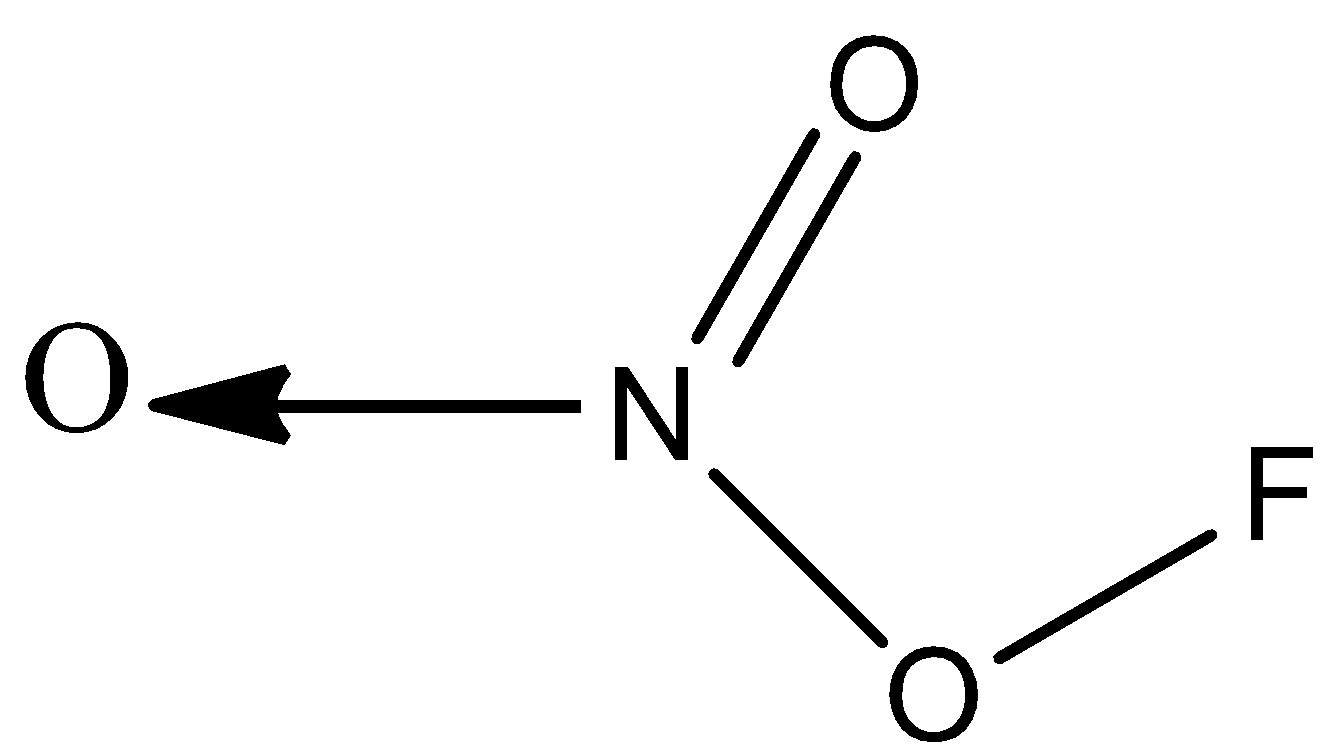
D)
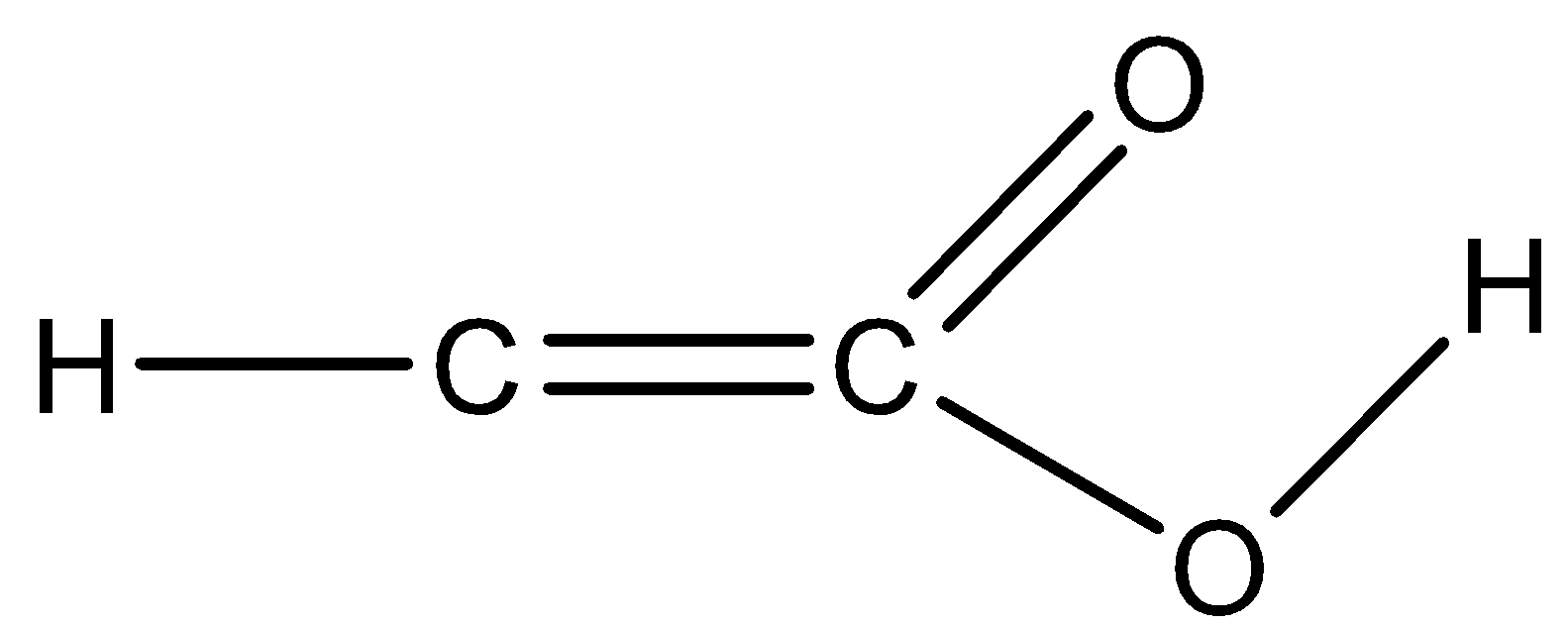




Answer
477.9k+ views
Hint: The degree of its combining ability with different atoms while it bureaucracy chemical substances or molecules is known as valence or valency. The combining ability or affinity of an atom of a given detail is decided by way of means of the range of hydrogen atoms that it combines with. In methane, the valency value of carbon is \[4\]; in ammonia, nitrogen valency value is $3$; in water, oxygen has a valence value of $2$; and in hydrogen chloride, chlorine has a valence of$1$. Chlorine, because it has a valence of one, maybe substituted for hydrogen.
Complete Answer:
To check the bond capacity of the following given compounds we need to check the valency of the central atom as well as other atoms also.
So in option A) the valency of the phosphorus atom is completely satisfied which is four thus it can be the correct answer for this question.
In option B) This option can also be correct since bonding pairs are two and the rest of oxygen valence electrons will participate as lone pairs, thus this option is also correct.
Option C) this option is also correct since the valency of nitrogen is satisfied; it forms a coordinate bond with oxygen, a double bond with oxygen, and a single bond with oxygen.
Option D) this option is incorrect as the valency of carbon is not satisfied for both the carbons. In one carbon it’s making $3$ bonds while the other carbon shows $5$bonds that are not the valency of carbon. Thus this option is not correct.
OPtion D is the correct answer.
Note:
If the outermost shell has eight electrons then the detail is stated to have a whole octet. By gaining, sharing, and dropping the electrons the atoms whole their outermost orbital and make an octet.
The capability of an atom is defined through the full variety of electrons lost, received or shared to finish its octet and it additionally determines the valency of the atom.
Complete Answer:
To check the bond capacity of the following given compounds we need to check the valency of the central atom as well as other atoms also.
So in option A) the valency of the phosphorus atom is completely satisfied which is four thus it can be the correct answer for this question.
In option B) This option can also be correct since bonding pairs are two and the rest of oxygen valence electrons will participate as lone pairs, thus this option is also correct.
Option C) this option is also correct since the valency of nitrogen is satisfied; it forms a coordinate bond with oxygen, a double bond with oxygen, and a single bond with oxygen.
Option D) this option is incorrect as the valency of carbon is not satisfied for both the carbons. In one carbon it’s making $3$ bonds while the other carbon shows $5$bonds that are not the valency of carbon. Thus this option is not correct.
OPtion D is the correct answer.
Note:
If the outermost shell has eight electrons then the detail is stated to have a whole octet. By gaining, sharing, and dropping the electrons the atoms whole their outermost orbital and make an octet.
The capability of an atom is defined through the full variety of electrons lost, received or shared to finish its octet and it additionally determines the valency of the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




