
Which conformer has higher energy in the Sawhorse projection formula of ethane and why?
Answer
588.9k+ views
Hint: Sawhorse is a simple method of representing three-dimensional formulae. There are 2 conformations of ethane: staggered and eclipsed. The staggered has more stability than the eclipsed form.
Complete step by step answer:
In an ethane ($C{{H}_{3}}-C{{H}_{3}}$) molecule, the two carbon atoms are connected by a single covalent bond while the remaining three valencies of each atom are satisfied by hydrogen atoms.
A sawhorse is a simple method of representing the three-dimensional formulae on paper. The molecule is viewed slightly from above. The bond between the two carbon atoms are placed diagonally and is slightly elongated for clarity. The Sawhorse representation for staggered and eclipsed conformation of ethane is shown below:
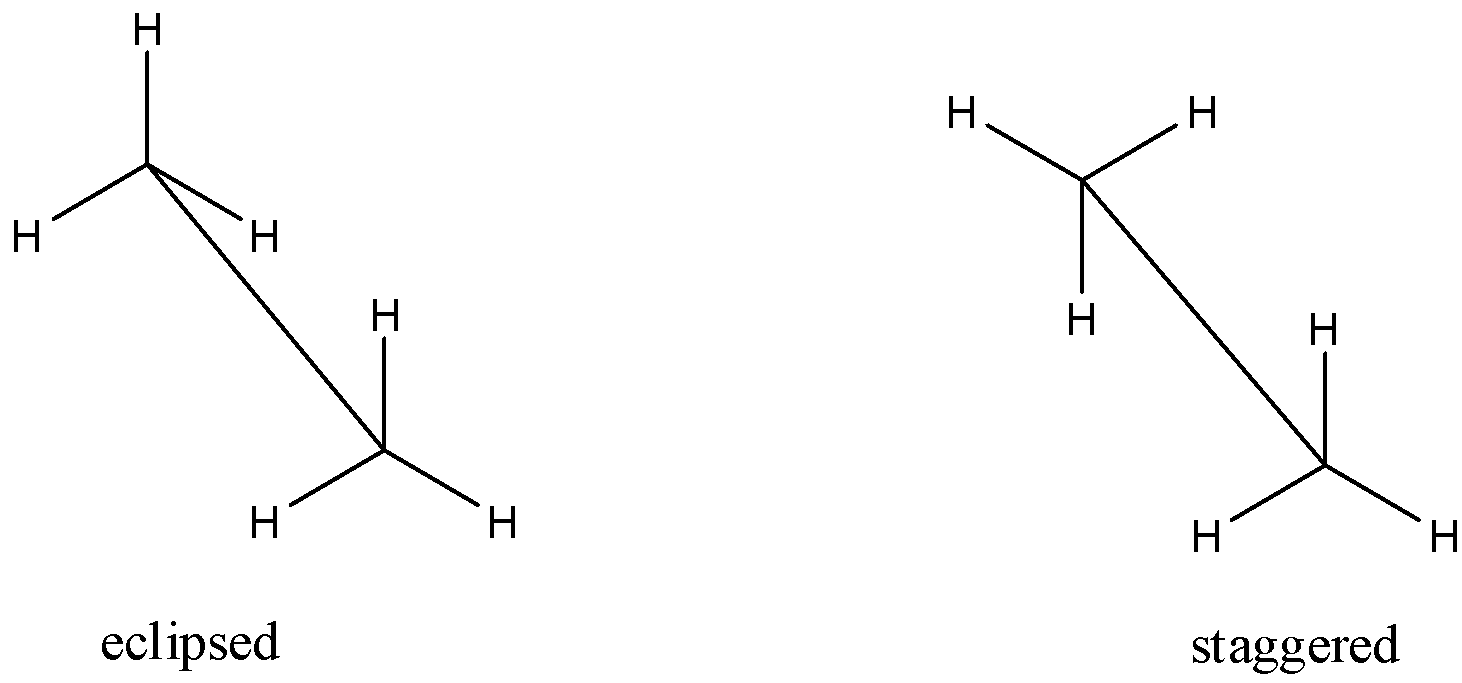
When the hydrogen atoms on the carbon are exactly placed between each of the hydrogen atoms on the back carbon, it is called staggered conformation. In other words, any two hydrogen atoms on adjacent carbon atoms are as far as possible. As a result, the repulsion between the electron clouds of $\sigma -bond$ of two non-bonded hydrogen atoms is minimal. On the other hand, if the hydrogen atoms on the back carbon are exactly placed behind each of the hydrogen atoms on the front carbon, it is called eclipsed conformation. In other words, the non-bonded hydrogen atoms are very close. As a result, the repulsion between the electron clouds of $\sigma -bond$ of two non-bonded hydrogen atoms is maximum. Because of this, the energy of the eclipsed conformation is higher than that of staggered conformation. This repulsive interaction is called torsional strain and it affects the stability of a conformation is called torsional strain. So, the eclipsed form has high energy and low stability and the staggered form has low energy and high stability.
Note: The two conformations are readily interconvertible. Due to this we cannot separate the mixture of conformers of ethane. However, most of the ethane molecules would exist in the staggered conformation because it has minimum energy and maximum stability.
Complete step by step answer:
In an ethane ($C{{H}_{3}}-C{{H}_{3}}$) molecule, the two carbon atoms are connected by a single covalent bond while the remaining three valencies of each atom are satisfied by hydrogen atoms.
A sawhorse is a simple method of representing the three-dimensional formulae on paper. The molecule is viewed slightly from above. The bond between the two carbon atoms are placed diagonally and is slightly elongated for clarity. The Sawhorse representation for staggered and eclipsed conformation of ethane is shown below:

When the hydrogen atoms on the carbon are exactly placed between each of the hydrogen atoms on the back carbon, it is called staggered conformation. In other words, any two hydrogen atoms on adjacent carbon atoms are as far as possible. As a result, the repulsion between the electron clouds of $\sigma -bond$ of two non-bonded hydrogen atoms is minimal. On the other hand, if the hydrogen atoms on the back carbon are exactly placed behind each of the hydrogen atoms on the front carbon, it is called eclipsed conformation. In other words, the non-bonded hydrogen atoms are very close. As a result, the repulsion between the electron clouds of $\sigma -bond$ of two non-bonded hydrogen atoms is maximum. Because of this, the energy of the eclipsed conformation is higher than that of staggered conformation. This repulsive interaction is called torsional strain and it affects the stability of a conformation is called torsional strain. So, the eclipsed form has high energy and low stability and the staggered form has low energy and high stability.
Note: The two conformations are readily interconvertible. Due to this we cannot separate the mixture of conformers of ethane. However, most of the ethane molecules would exist in the staggered conformation because it has minimum energy and maximum stability.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




