
Which compound does not have tautomerism?
A.\[C{H_3} - COC{H_2}COC{H_3}\]
B.\[{C_6}{H_5} - CH = N - OH\]
C.
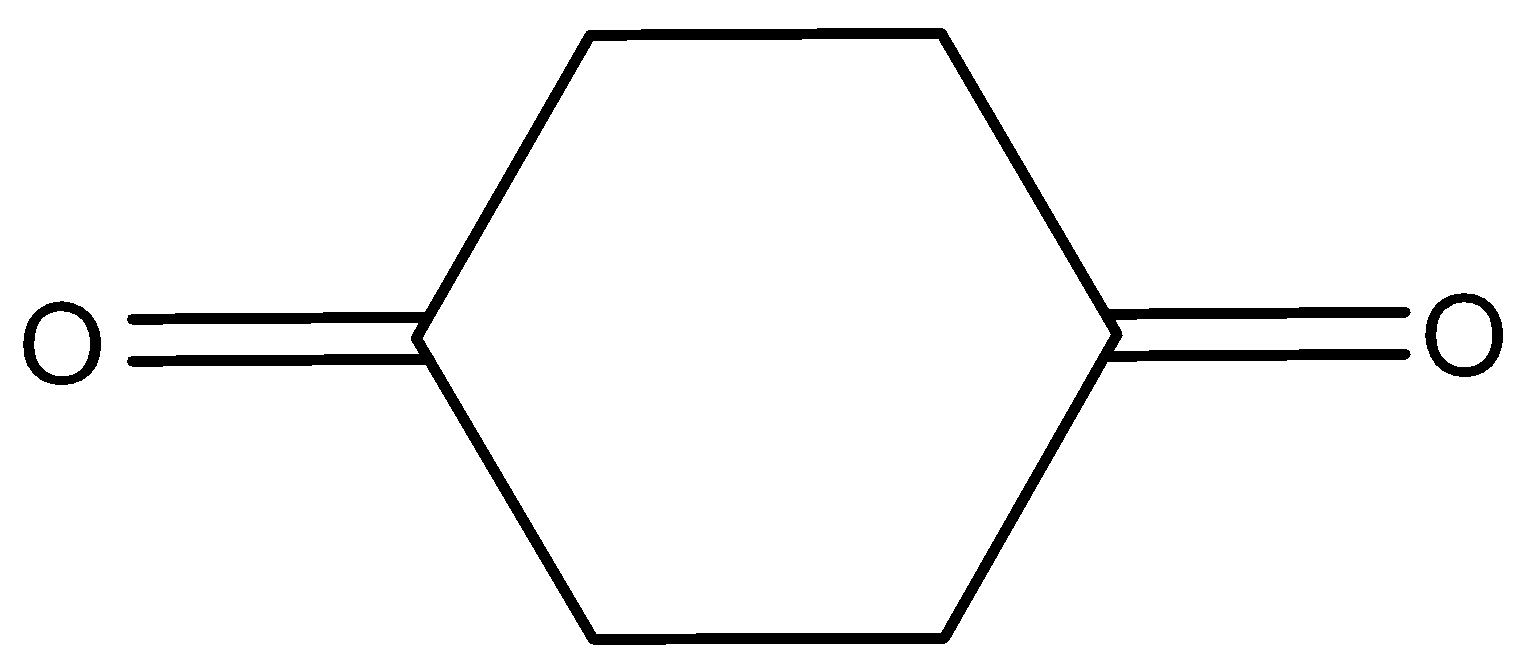
D.
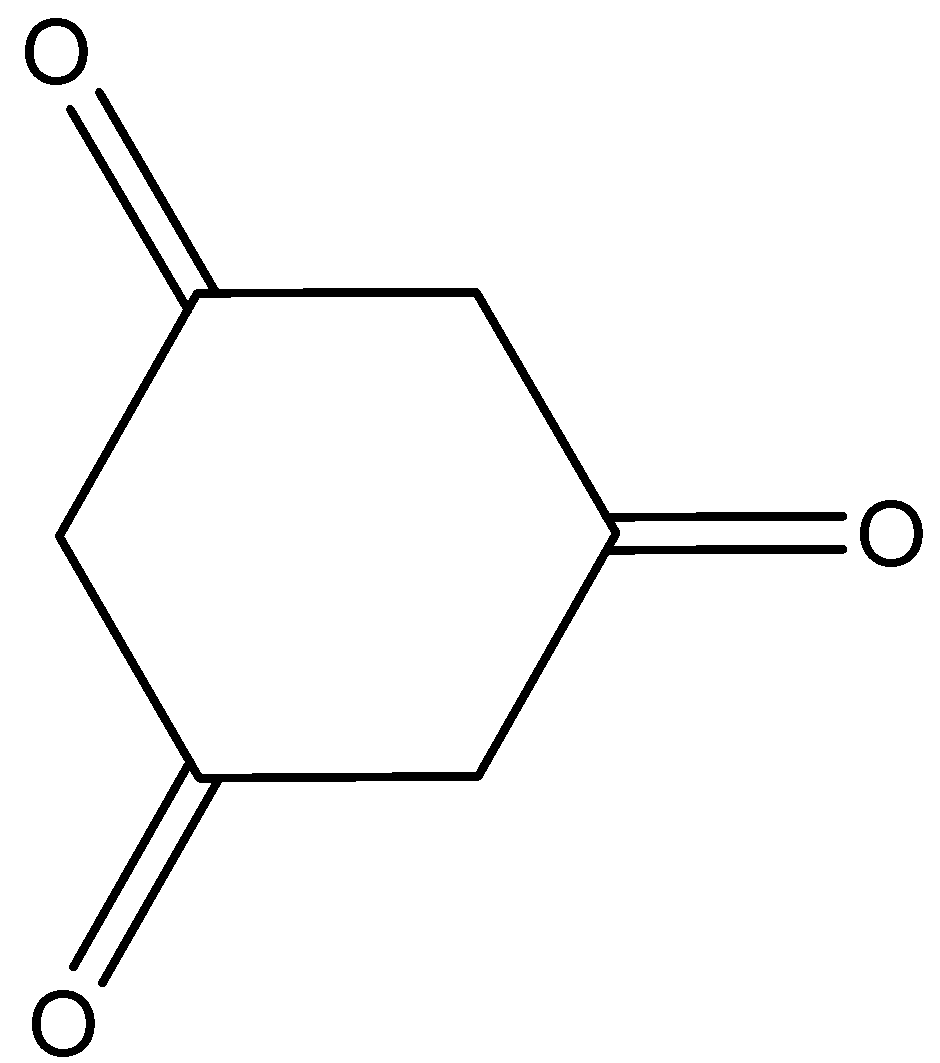


Answer
478.5k+ views
Hint: The compounds with different positions of protons and electrons are known as tautomers. And these are the constitutional isomers. Here, the carbon Skeleton of the compound will not change. A reaction that takes place by the simple transfer of protons in an intramolecular manner is known as tautomerism. And the isomer of phenol is an important example of tautomers.
Complete answer:
Acetyl acetone is a chemical compound having the formula\[C{H_3} - COC{H_2}COC{H_3}\]and it is classified as \[1,3 - \]diketone. It exhibits a tautomer in equilibrium condition and that is, \[C{H_3}\left( O \right)CH = \left( {OH} \right)C{H_3}\]. And this tautomer is interconverted very quickly. That is,
\[C{H_3} - COC{H_2}COC{H_3} \rightleftharpoons C{H_3}\left( O \right)CH = \left( {OH} \right)C{H_3}\]
Hence, option (A) is incorrect.
The benzaldehyde oxime is an organic compound having the chemical formula, \[{C_6}{H_5} - CH = N - OH\]. And it will show the tautomerism and that is,
\[{C_6}{H_5} - CH = N - OH \rightleftharpoons {C_6}{H_5} - C{H_2} - N = O\]
Hence, the option (B) is incorrect.
Here, the cyclohexane\[ - 1,4 - \]dione will not show the tautomerism. Because here the alpha hydrogen is attached to the \[s{p^2}\]hybridized carbon atom and it is very difficult to remove. Therefore, it does not show tautomerism.
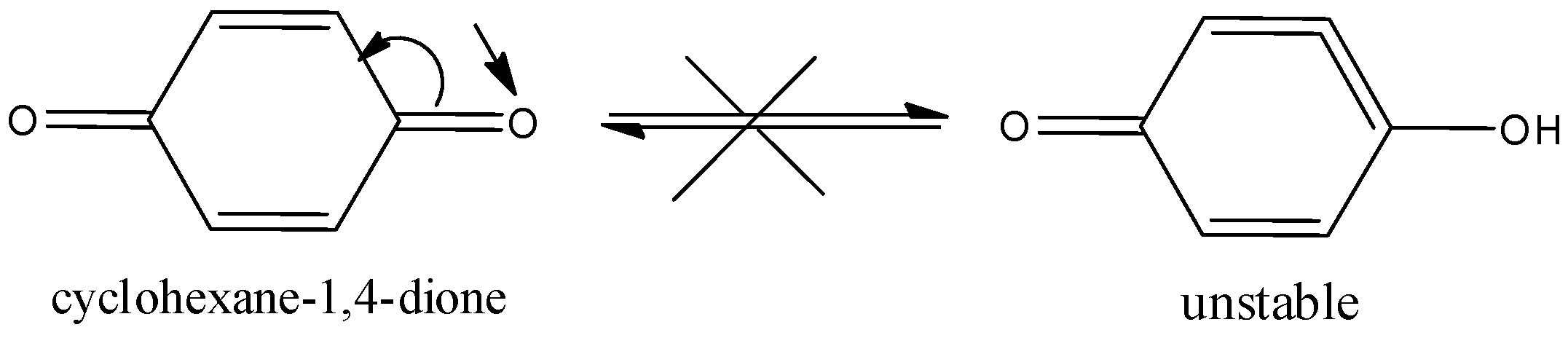
Hence, option (C) is correct.
Here, cyclohexane\[ - 1,3,5 - \]trione shows the tautomerism. And that is,
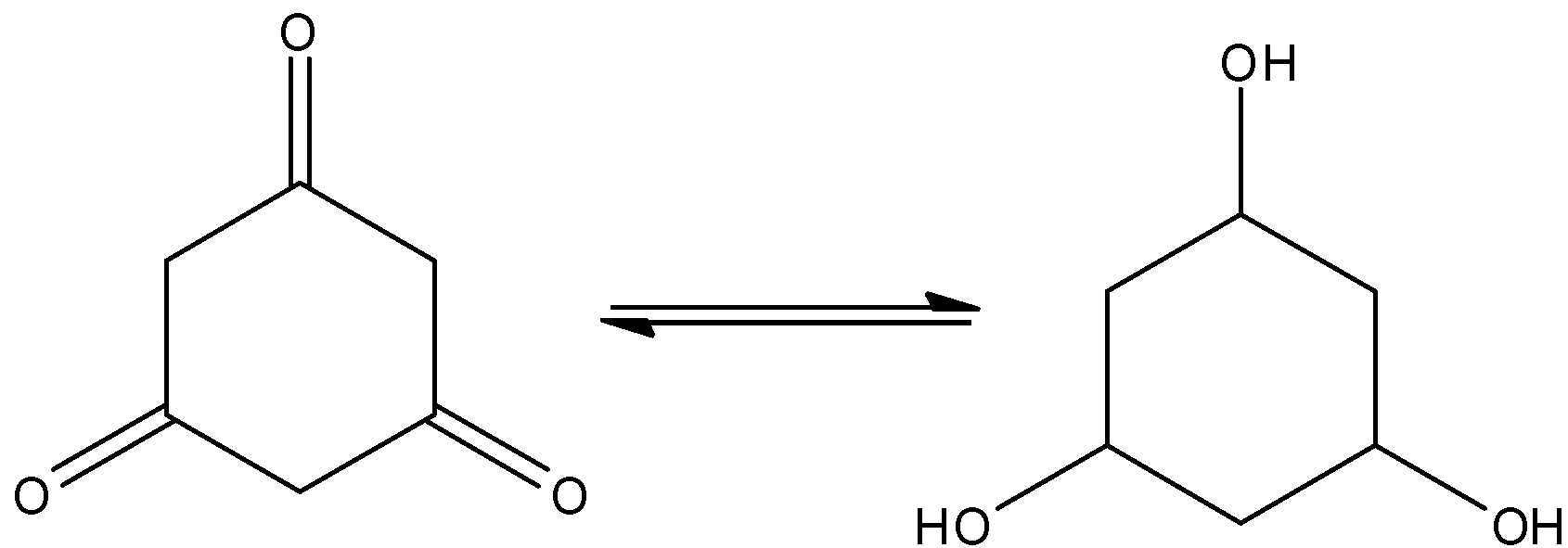
Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
If the compound does not contain the alpha hydrogen atom or the alpha hydrogen is attached to the\[s{p^2}\] hybridized carbon atom, then that compound will not show the tautomerism. Because the alpha hydrogen is very difficult to remove from the \[s{p^2}\]hybridized carbon atom. For another example, in the case of benzaldehyde, which does not contain \[s{p^2}\]hybridized carbon atoms. Therefore, it will not show tautomerism.
Complete answer:
Acetyl acetone is a chemical compound having the formula\[C{H_3} - COC{H_2}COC{H_3}\]and it is classified as \[1,3 - \]diketone. It exhibits a tautomer in equilibrium condition and that is, \[C{H_3}\left( O \right)CH = \left( {OH} \right)C{H_3}\]. And this tautomer is interconverted very quickly. That is,
\[C{H_3} - COC{H_2}COC{H_3} \rightleftharpoons C{H_3}\left( O \right)CH = \left( {OH} \right)C{H_3}\]
Hence, option (A) is incorrect.
The benzaldehyde oxime is an organic compound having the chemical formula, \[{C_6}{H_5} - CH = N - OH\]. And it will show the tautomerism and that is,
\[{C_6}{H_5} - CH = N - OH \rightleftharpoons {C_6}{H_5} - C{H_2} - N = O\]
Hence, the option (B) is incorrect.
Here, the cyclohexane\[ - 1,4 - \]dione will not show the tautomerism. Because here the alpha hydrogen is attached to the \[s{p^2}\]hybridized carbon atom and it is very difficult to remove. Therefore, it does not show tautomerism.

Hence, option (C) is correct.
Here, cyclohexane\[ - 1,3,5 - \]trione shows the tautomerism. And that is,

Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
If the compound does not contain the alpha hydrogen atom or the alpha hydrogen is attached to the\[s{p^2}\] hybridized carbon atom, then that compound will not show the tautomerism. Because the alpha hydrogen is very difficult to remove from the \[s{p^2}\]hybridized carbon atom. For another example, in the case of benzaldehyde, which does not contain \[s{p^2}\]hybridized carbon atoms. Therefore, it will not show tautomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




