
Which are true statements among the following?
1.\[P{H_5}\] and \[BiC{l_5}\] do not exist
2.P, d bonds are present in \[S{O_2}\]
3.Electrons travel at the speed of light
4.\[Se{F_4}\] and \[C{H_4}\] have the same shape
5.\[I_3^ + \] has bent geometry
A.1,3
B.1,2,5
C.1,3,5
D.1,2,4
Answer
582.6k+ views
Hint: In order to solve this question, we must individually solve all the statements to determine the incorrect ones from them. Then in the end, we can group all these incorrect statements together and choose the corresponding option
Complete Step-by-Step Answer:
1. \[P{H_5}\] and \[BiC{l_5}\] do not exist:
\[P{H_5}\] as a compound, ceases to exist because p orbitals of phosphorus interact with s orbitals of hydrogen. A bond formed in this hybridized state is not stable. Hence it does not exist. A similar situation happens with \[BiC{l_5}\] . The +5-oxidation state of \[BiC{l_5}\] has a very low stability in comparison to its +3-oxidation state. Hence, even \[BiC{l_5}\] does not exist.
Hence, this statement is true.
2.P, d bonds are present in \[S{O_2}\] :
To answer this, it is better if we draw a Lewis Structure of \[S{O_2}\] .
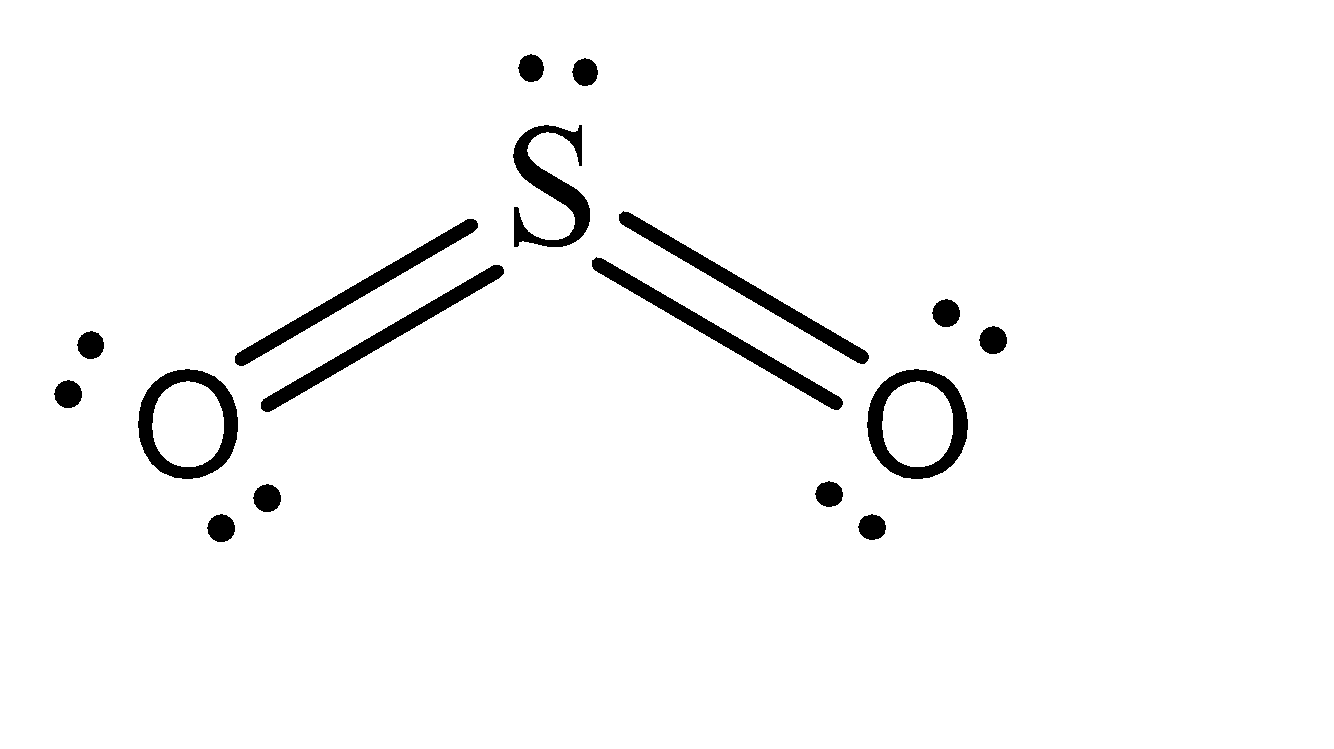
As we can see, 2 pi bonds are formed in the case of \[S{O_2}\] . Now the nature of these bonds can be determined by understanding the hybridization. The unpaired electrons of sulphur reside in the 3p and 3d hybridized orbitals, while the pi bonds are formed by interacting with oxygen’s 2p orbitals. Hence, the two pi bonds formed consist of one \[p-p\] pi bond and one \[p-d\] pi bond.
Hence, this statement is true
3.Electrons travel at the speed of light:
No entity which has a mass can travel at the speed of light. Electrons have a mass. Hence, it cannot travel at the speed of light.
Hence, this statement is false.
4. \[Se{F_4}\] and \[C{H_4}\] have the same shape:
To understand this statement, let us draw the structures of the two compounds.
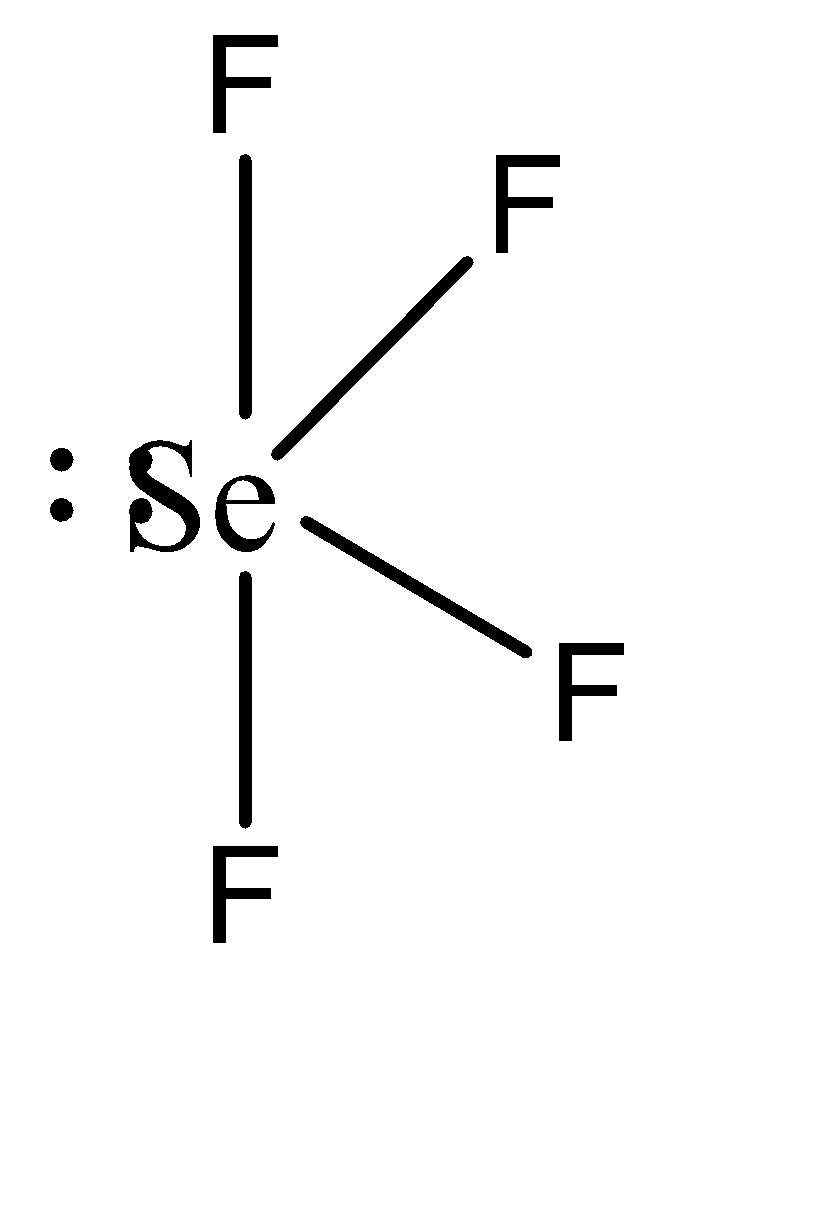

We can observe that \[Se{F_4}\] has a seesaw shape while \[C{H_4}\] has a tetrahedral shape. In \[Se{F_4}\] , the repulsion caused by the lone pair forces the other atoms to move away from it, resulting in the seesaw shape. Similarly, equivalent electron repulsion from all the hydrogen atoms in \[C{H_4}\] causes them to form a uniform tetrahedral geometry.
Hence, the given statement is false.
5. \[I_3^ + \] has bent geometry:
This statement is true because \[I_3^ + \] does have a bent geometry, because of its trigonal bi-pyramidal structure
Hence, statements 1, 2 and 5 are correct
Hence, Option B is the correct option.
Note: While solving for the last statement, students may often think about the structure of \[I_3^ + \] as a linear structure while drawing the Lewis Structure. But in reality, due to the presence of lone pairs on all three iodine atoms, it forms a trigonal bipyramidal structure.
Complete Step-by-Step Answer:
1. \[P{H_5}\] and \[BiC{l_5}\] do not exist:
\[P{H_5}\] as a compound, ceases to exist because p orbitals of phosphorus interact with s orbitals of hydrogen. A bond formed in this hybridized state is not stable. Hence it does not exist. A similar situation happens with \[BiC{l_5}\] . The +5-oxidation state of \[BiC{l_5}\] has a very low stability in comparison to its +3-oxidation state. Hence, even \[BiC{l_5}\] does not exist.
Hence, this statement is true.
2.P, d bonds are present in \[S{O_2}\] :
To answer this, it is better if we draw a Lewis Structure of \[S{O_2}\] .

As we can see, 2 pi bonds are formed in the case of \[S{O_2}\] . Now the nature of these bonds can be determined by understanding the hybridization. The unpaired electrons of sulphur reside in the 3p and 3d hybridized orbitals, while the pi bonds are formed by interacting with oxygen’s 2p orbitals. Hence, the two pi bonds formed consist of one \[p-p\] pi bond and one \[p-d\] pi bond.
Hence, this statement is true
3.Electrons travel at the speed of light:
No entity which has a mass can travel at the speed of light. Electrons have a mass. Hence, it cannot travel at the speed of light.
Hence, this statement is false.
4. \[Se{F_4}\] and \[C{H_4}\] have the same shape:
To understand this statement, let us draw the structures of the two compounds.


We can observe that \[Se{F_4}\] has a seesaw shape while \[C{H_4}\] has a tetrahedral shape. In \[Se{F_4}\] , the repulsion caused by the lone pair forces the other atoms to move away from it, resulting in the seesaw shape. Similarly, equivalent electron repulsion from all the hydrogen atoms in \[C{H_4}\] causes them to form a uniform tetrahedral geometry.
Hence, the given statement is false.
5. \[I_3^ + \] has bent geometry:
This statement is true because \[I_3^ + \] does have a bent geometry, because of its trigonal bi-pyramidal structure
Hence, statements 1, 2 and 5 are correct
Hence, Option B is the correct option.
Note: While solving for the last statement, students may often think about the structure of \[I_3^ + \] as a linear structure while drawing the Lewis Structure. But in reality, due to the presence of lone pairs on all three iodine atoms, it forms a trigonal bipyramidal structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




