
Which among the following is likely to show geometrical isomerism?
A) $C{{H}_{3}}CH=NOH$
B)$C{{H}_{3}}CH=C{{H}_{2}}$
C) $C{{H}_{2}}=CH=CH=CC{{l}_{2}}$
D) $C{{H}_{3}}C(Cl)=C{{(C{{H}_{3}})}_{2}}$
Answer
569.7k+ views
Hint: Geometrical isomers are those structures which possess the same molecular formula but they will have different directional arrangement of specific groups in the molecule near the C atom with respect to the study.
Complete step by step answer:
So in the question we are asked, is, to select the correct option which possesses geometrical isomerism from the given options.
Isomerism-It is the phenomenon in which the two structures are having the same molecular formula but differ by either position of bonds or functional groups, bond connectivity, arrangement of groups in space etc.
So now let’s talk about geometrical isomers-
Geometrical isomers are a type of stereoisomers i.e. the structures possess the same molecular formulae and the bond connectivity are also similar, but there is difference in the arrangement of specific groups among the C, mainly among the C with double bonds.
Geometrical isomers are mainly formed due to the restricted rotation of molecules and the restricted rotation is due to the presence of a double bond. Geometrical isomers are identified if there are different groups nearby the C with the double bond and if the groups are arranged in different directions in the space.
Geometrical isomers are of two types- cis and trans isomers.
Cis isomers consists of the same group present in the same side of the C double bonded atom and for trans the groups will be oppositely placed.
Now let us solve the problem,
Option (A), $C{{H}_{3}}CH=NOH$ and has the structure
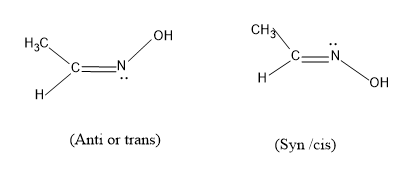
Here the groups present in this atom can be arranged in such fashion in space, the trans will have the –OH group upwards and in cis it is facing downwards and this ,molecule satisfies the condition of showing geometrical isomerism, so this ,may be the answer.
Now option (B), $C{{H}_{3}}CH=C{{H}_{2}}$ and has a structure

Here in the structure the right side if the C=C is having the same group as the substituent i.e.H. Hence geometrical isomers are not possible for this structure.
For option (C), $C{{H}_{2}}=CH=CH=CC{{l}_{2}}$
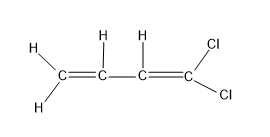
Here there are two C=C present in structure, but both the C=C has the similar groups as the substituents, hence the geometrical isomer structures are not possible for this molecule.
Option (D), $C{{H}_{3}}C(Cl)=C{{(C{{H}_{3}})}_{2}}$
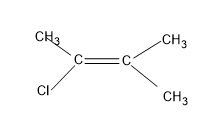
Here, the right side of the C=C of the molecule is having two methyl attached to it as both are similar groups, geometrical isomers are not possible.
So we can conclude that only option (A) satisfies the criteria of geometrical isomers and hence the correct option is option (A).
Note: The main criteria or prerequisite to show the phenomenon of geometrical isomers is that the C=C should possess different groups in the both sides of the atom.
And we should always restrict the rotation of C=C, so that we could get geometrical isomeric structures.
Complete step by step answer:
So in the question we are asked, is, to select the correct option which possesses geometrical isomerism from the given options.
Isomerism-It is the phenomenon in which the two structures are having the same molecular formula but differ by either position of bonds or functional groups, bond connectivity, arrangement of groups in space etc.
So now let’s talk about geometrical isomers-
Geometrical isomers are a type of stereoisomers i.e. the structures possess the same molecular formulae and the bond connectivity are also similar, but there is difference in the arrangement of specific groups among the C, mainly among the C with double bonds.
Geometrical isomers are mainly formed due to the restricted rotation of molecules and the restricted rotation is due to the presence of a double bond. Geometrical isomers are identified if there are different groups nearby the C with the double bond and if the groups are arranged in different directions in the space.
Geometrical isomers are of two types- cis and trans isomers.
Cis isomers consists of the same group present in the same side of the C double bonded atom and for trans the groups will be oppositely placed.
Now let us solve the problem,
Option (A), $C{{H}_{3}}CH=NOH$ and has the structure

Here the groups present in this atom can be arranged in such fashion in space, the trans will have the –OH group upwards and in cis it is facing downwards and this ,molecule satisfies the condition of showing geometrical isomerism, so this ,may be the answer.
Now option (B), $C{{H}_{3}}CH=C{{H}_{2}}$ and has a structure

Here in the structure the right side if the C=C is having the same group as the substituent i.e.H. Hence geometrical isomers are not possible for this structure.
For option (C), $C{{H}_{2}}=CH=CH=CC{{l}_{2}}$

Here there are two C=C present in structure, but both the C=C has the similar groups as the substituents, hence the geometrical isomer structures are not possible for this molecule.
Option (D), $C{{H}_{3}}C(Cl)=C{{(C{{H}_{3}})}_{2}}$

Here, the right side of the C=C of the molecule is having two methyl attached to it as both are similar groups, geometrical isomers are not possible.
So we can conclude that only option (A) satisfies the criteria of geometrical isomers and hence the correct option is option (A).
Note: The main criteria or prerequisite to show the phenomenon of geometrical isomers is that the C=C should possess different groups in the both sides of the atom.
And we should always restrict the rotation of C=C, so that we could get geometrical isomeric structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




