
Which among the following is Claus formula of benzene?
A.
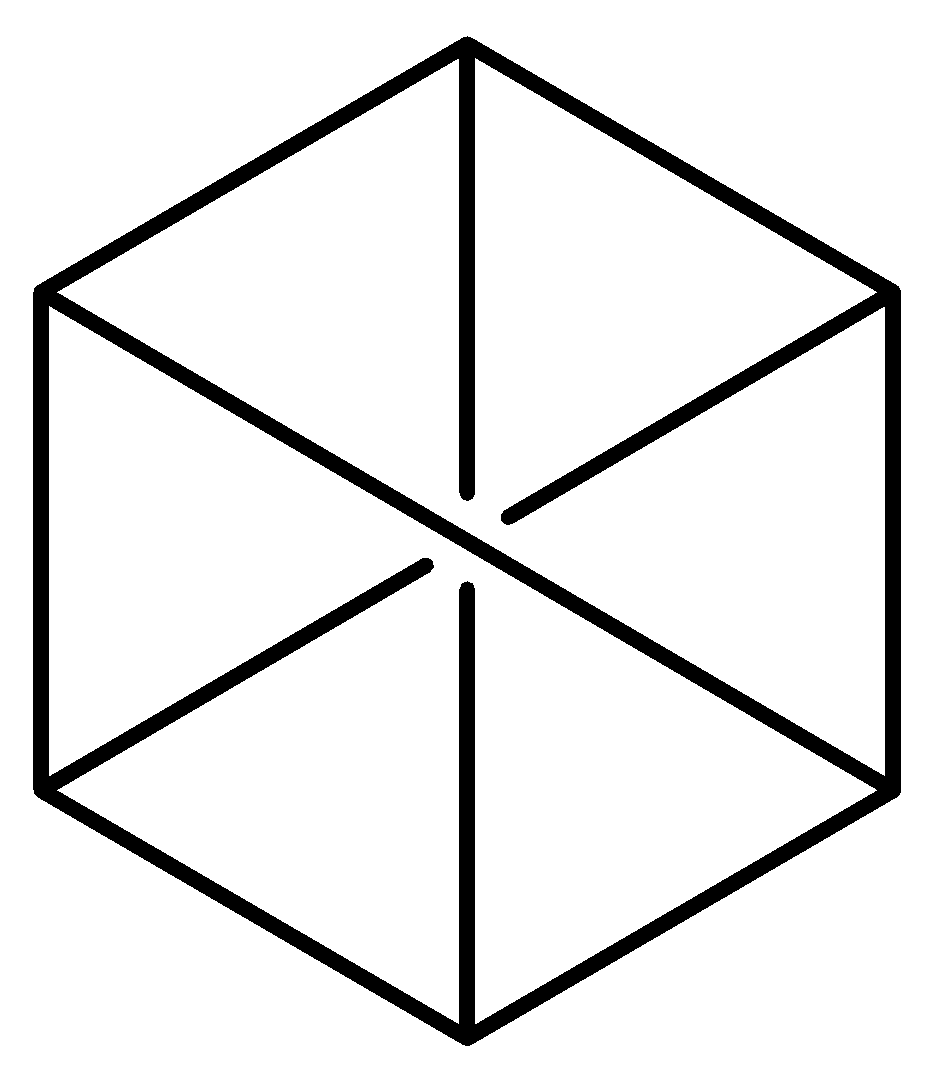
B.
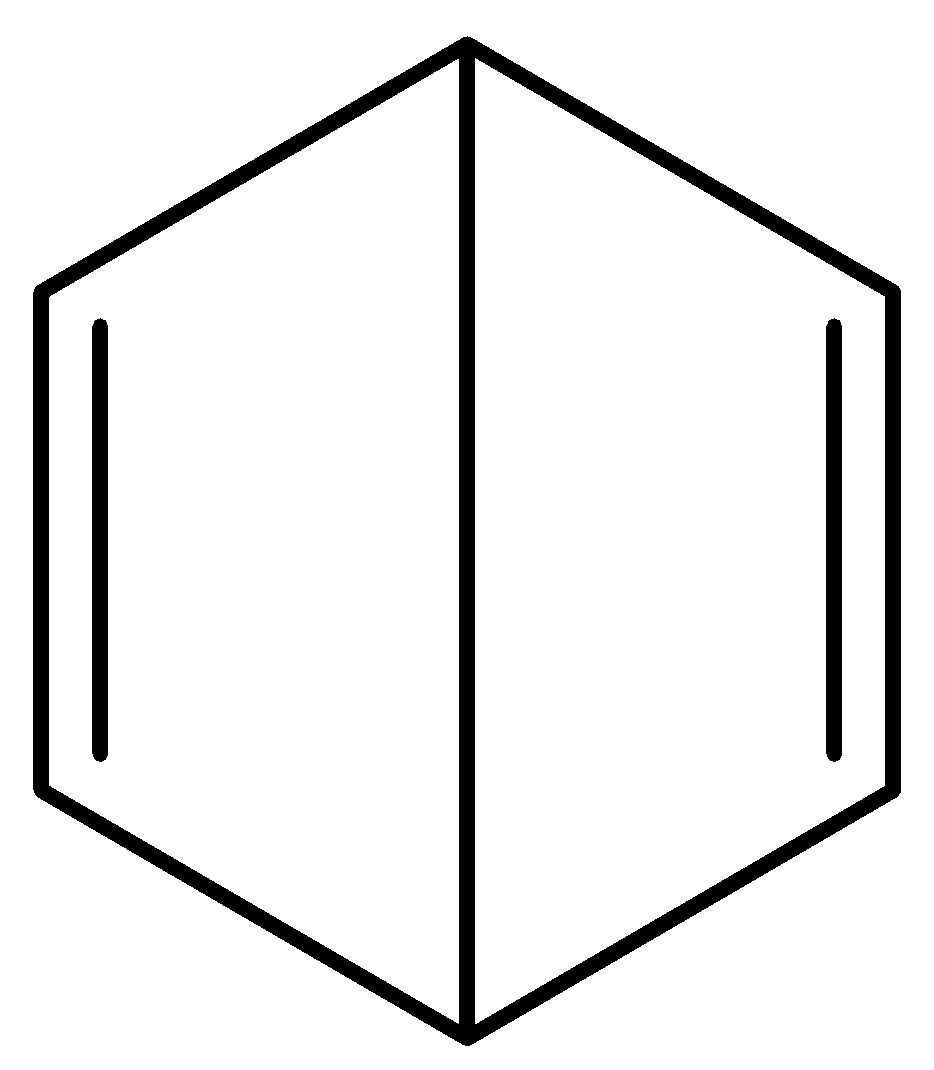
C.
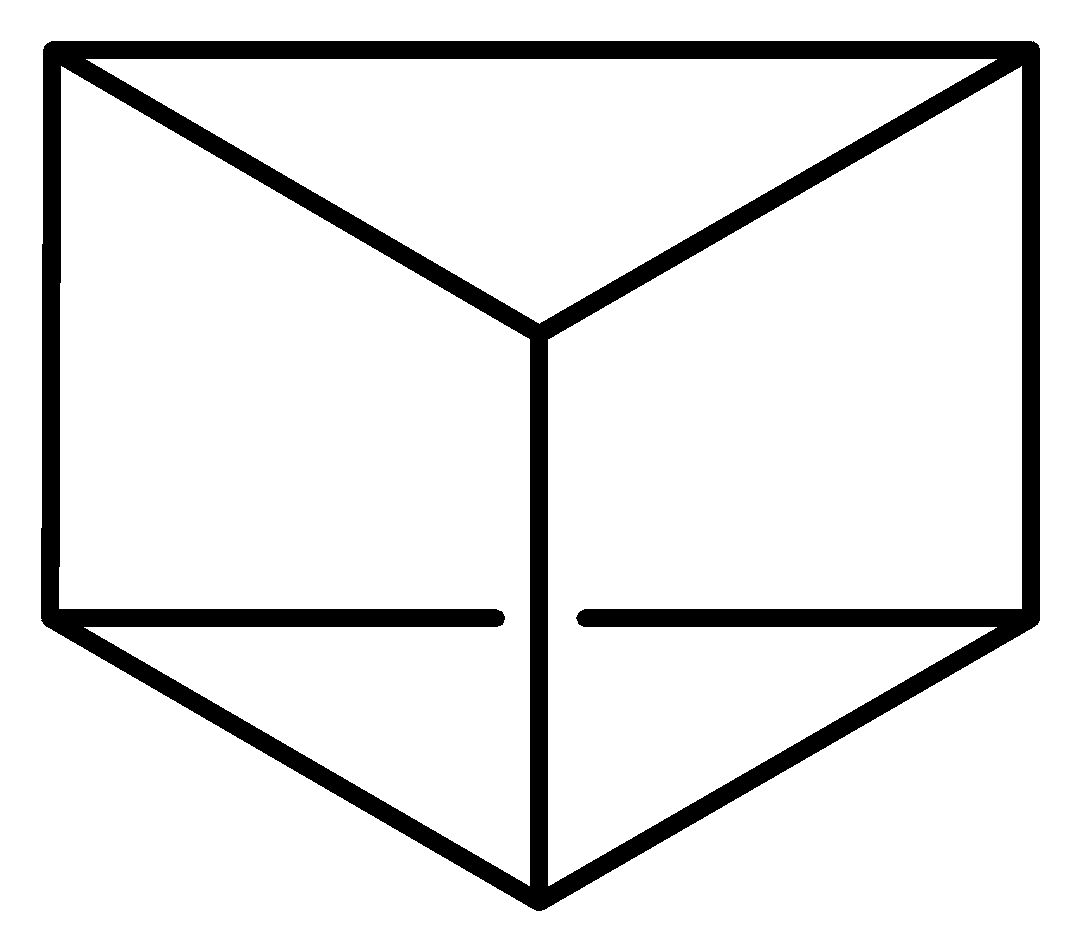
D.
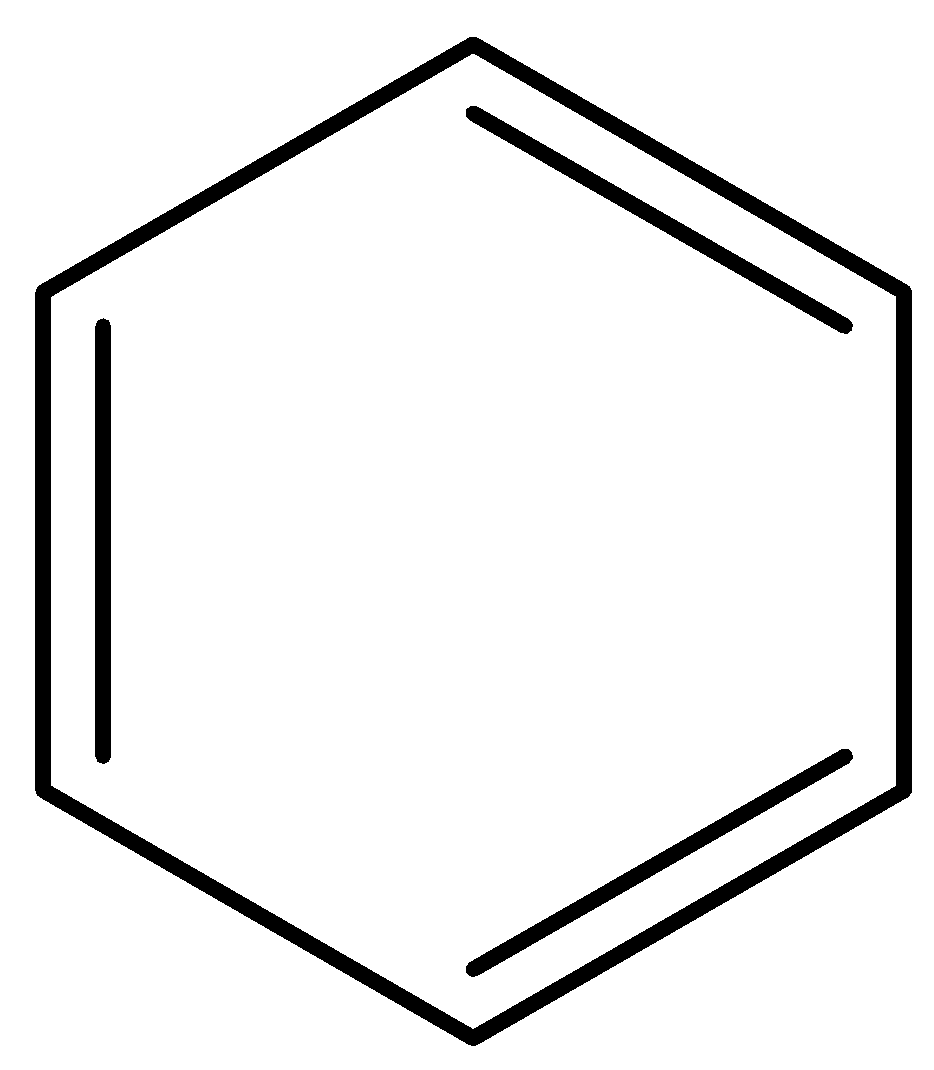




Answer
576.6k+ views
Hint: Claus benzene molecule is a hexagon with six carbon (C) atoms with a hydrogen (H) atom positioned at the corners, with each carbon connected to its two ortho carbons (the nearest carbons) and the one para carbon connected diametrically.
Complete step by step answer:
Claus benzene $\left( {{C_6}{H_6}} \right)$ is a hypothetical hydrocarbon and it is an isomer of benzene. It was proposed by a German chemist, Adolf Karl Ludwig Claus in the year 1867 as a possible structure for benzene at a time when there was still a debate on the structure of benzene. In his model, the six carbon (C) atoms of benzene form a hexagonal shape with a hydrogen atom positioned to every corner. The opposite corners of the hexagon are connected by single bonds to preserve 4 valances for carbon atoms i.e. each carbon is connected to its two ortho carbons and the one para carbon connected diametrically. High strain energy makes Claus benezene’s synthesis impossible. Although it is often referred to alongside Dewar benzene which is option (B) and Prismane, it is not possible to synthesise it, which Dewar benzene and Prismane can be.
Therefore, the correct answer is option (A).
Note: It took many years of research before the three postulated structures of benzene, by Albert Ladenburg in 1869, August Kekulé in 1865 and by Claus in 1867 found their place in organic chemistry. Ladenburg's prismane and Claus' benzene were both proven to be wrong, on the other hand, the prismane was synthesized in 1973. The calculations showed that the synthesis of Claus' benzene is impossible.
Complete step by step answer:
Claus benzene $\left( {{C_6}{H_6}} \right)$ is a hypothetical hydrocarbon and it is an isomer of benzene. It was proposed by a German chemist, Adolf Karl Ludwig Claus in the year 1867 as a possible structure for benzene at a time when there was still a debate on the structure of benzene. In his model, the six carbon (C) atoms of benzene form a hexagonal shape with a hydrogen atom positioned to every corner. The opposite corners of the hexagon are connected by single bonds to preserve 4 valances for carbon atoms i.e. each carbon is connected to its two ortho carbons and the one para carbon connected diametrically. High strain energy makes Claus benezene’s synthesis impossible. Although it is often referred to alongside Dewar benzene which is option (B) and Prismane, it is not possible to synthesise it, which Dewar benzene and Prismane can be.
Therefore, the correct answer is option (A).
Note: It took many years of research before the three postulated structures of benzene, by Albert Ladenburg in 1869, August Kekulé in 1865 and by Claus in 1867 found their place in organic chemistry. Ladenburg's prismane and Claus' benzene were both proven to be wrong, on the other hand, the prismane was synthesized in 1973. The calculations showed that the synthesis of Claus' benzene is impossible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




