
Which among the following is a synthetic polymer? (a) Rubber (b) Teflon (c) DNA (d) Wool
Answer
506.7k+ views
Hint: Polymers are macromolecules consisting of many repeating units. Natural and synthetic polymers are the two types of polymers. Synthetic polymers are made by man.
Complete answer:
Synthetic polymers are those polymers which are made by man. Amongst the given examples, Teflon is the synthetic polymer while the other i.e. rubber, wool and DNA are natural polymers. Rubber and wool are derived from plants while DNA is present in animals. The polymerization reaction for the synthesis of Teflon is given as:
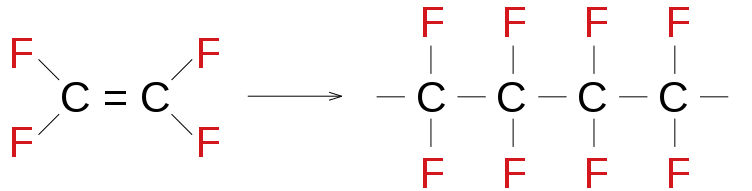
In the above reaction, tetrafluoroethylene acts as a monomer and leads to the formation of polytetrafluoroethylene (PTFE) as a polymer product. Polytetrafluoroethylene is called teflon. It is an example of an additional polymer which is formed via chain growth (free-radical) polymerization.
In all, we can say that among the following polymers, teflon is a synthetic polymer.
So, the correct answer is “Option b”.
Additional information: Teflon has numerous applications. For instance: Teflon is used as a non-sticky coating material on the utensils which is used for cooking purposes. Besides, Teflon is also used for coating the wires. Moreover, Teflon is a thermoplastic polymer which becomes mold on heating and solidifies upon cooling.
Note:
It is important to note that Teflon is the synthetic polymer while the other three i.e. rubber, wool and DNA are natural polymers. Teflon is called polytetrafluoroethylene in which the monomeric unit is tetrafluoroethylene. Teflon is an example of an additional polymer which is formed via chain growth (free-radical) polymerization.
Complete answer:
Synthetic polymers are those polymers which are made by man. Amongst the given examples, Teflon is the synthetic polymer while the other i.e. rubber, wool and DNA are natural polymers. Rubber and wool are derived from plants while DNA is present in animals. The polymerization reaction for the synthesis of Teflon is given as:

In the above reaction, tetrafluoroethylene acts as a monomer and leads to the formation of polytetrafluoroethylene (PTFE) as a polymer product. Polytetrafluoroethylene is called teflon. It is an example of an additional polymer which is formed via chain growth (free-radical) polymerization.
In all, we can say that among the following polymers, teflon is a synthetic polymer.
So, the correct answer is “Option b”.
Additional information: Teflon has numerous applications. For instance: Teflon is used as a non-sticky coating material on the utensils which is used for cooking purposes. Besides, Teflon is also used for coating the wires. Moreover, Teflon is a thermoplastic polymer which becomes mold on heating and solidifies upon cooling.
Note:
It is important to note that Teflon is the synthetic polymer while the other three i.e. rubber, wool and DNA are natural polymers. Teflon is called polytetrafluoroethylene in which the monomeric unit is tetrafluoroethylene. Teflon is an example of an additional polymer which is formed via chain growth (free-radical) polymerization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




