
Which among the following is a polar molecule?
\[
A.{\text{ }}B{H_3} \\
B.{\text{ }}N{F_3} \\
C.{\text{ }}{C_2}{H_6} \\
D.{\text{ }}S{F_6} \\
E.{\text{ }}CC{l_4} \\
\]
Answer
598.2k+ views
Hint: In order to solve the given problem and find the polar molecule amongst the given options we will first understand the concept of polarity of molecules and also understand the concept behind the classification of a molecule as polar or nonpolar on the basis of their dipole moment.
Complete step by step solution:
Polarity is a division of the electric charge which leads to a molecule or its chemical groups having an electric dipole moment with a negative charged end and a positively charged end. Regardless of a difference in electronegativity between the bound atoms, polar molecules may have polar bonds.
Polarity of any given molecule is found on the basis of the dipole moment of the molecule which is found out by the visualisation of positive and negative charge of the molecule.
On the basis of structure of the molecule the molecules are of two types:- symmetrical and unsymmetrical molecules.
The dipole moment of symmetrical molecule is zero as the net charge of in the molecule is cancelled out so these molecules does not has polarity
The bond dipole moment uses the concept of the electric dipole moment to calculate a chemical bond's polarity within a molecule. It occurs whenever the positives and negative charges are separated.
But the dipole moment of a non symmetrical molecule is not zero as the charge does not cancel out each other so unsymmetrical molecules are generally polar.
Amongst the given options the structure of each of the molecules other than $N{F_3}$ is non symmetrical and so the dipole moment of all the other molecules is zero.
Structure of $N{F_3}$
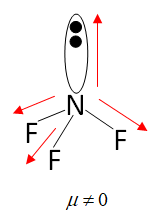
As we can see in the diagram the structure of $N{F_3}$ is non symmetrical due to the presence of a lone pair. So the dipole moment of $N{F_3}$ is not zero so the molecule is polar.
Hence, $N{F_3}$ is a polar molecule.
So, the correct answer is option B.
Note: In order to solve such problems related to polarity of the molecule. Students must be aware of the concept of dipole moment and also should know the structure of the molecules. $N{F_3}$ or nitrogen trifluoride can be used in relatively few manufacturing processes. This is used mainly in the manufacture of semiconductors and LCD (Liquid Crystal Display) displays, and certain types of solar panels and chemical lasers.
Complete step by step solution:
Polarity is a division of the electric charge which leads to a molecule or its chemical groups having an electric dipole moment with a negative charged end and a positively charged end. Regardless of a difference in electronegativity between the bound atoms, polar molecules may have polar bonds.
Polarity of any given molecule is found on the basis of the dipole moment of the molecule which is found out by the visualisation of positive and negative charge of the molecule.
On the basis of structure of the molecule the molecules are of two types:- symmetrical and unsymmetrical molecules.
The dipole moment of symmetrical molecule is zero as the net charge of in the molecule is cancelled out so these molecules does not has polarity
The bond dipole moment uses the concept of the electric dipole moment to calculate a chemical bond's polarity within a molecule. It occurs whenever the positives and negative charges are separated.
But the dipole moment of a non symmetrical molecule is not zero as the charge does not cancel out each other so unsymmetrical molecules are generally polar.
Amongst the given options the structure of each of the molecules other than $N{F_3}$ is non symmetrical and so the dipole moment of all the other molecules is zero.
Structure of $N{F_3}$

As we can see in the diagram the structure of $N{F_3}$ is non symmetrical due to the presence of a lone pair. So the dipole moment of $N{F_3}$ is not zero so the molecule is polar.
Hence, $N{F_3}$ is a polar molecule.
So, the correct answer is option B.
Note: In order to solve such problems related to polarity of the molecule. Students must be aware of the concept of dipole moment and also should know the structure of the molecules. $N{F_3}$ or nitrogen trifluoride can be used in relatively few manufacturing processes. This is used mainly in the manufacture of semiconductors and LCD (Liquid Crystal Display) displays, and certain types of solar panels and chemical lasers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




