
Which among the following has the highest boiling point?
A. \[{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl}}\]
B. ${\left( {{{C}}{{{H}}_3}} \right)_2}{{CHC}}{{{H}}_2}{{Cl}}$
C. ${\left( {{{C}}{{{H}}_3}} \right)_3}{{C}} - {{Cl}}$
D. None of the above
Answer
558.9k+ views
Hint: The given compounds are simply hydrocarbons substituted with halogens. Thus they are called alkyl halides. Some of them are branched. Generally, molecules which do not have branching increase their boiling point with respect to their increase in the molecular weight.
Complete step by step answer:
The structures with their names of the given compounds are given below:
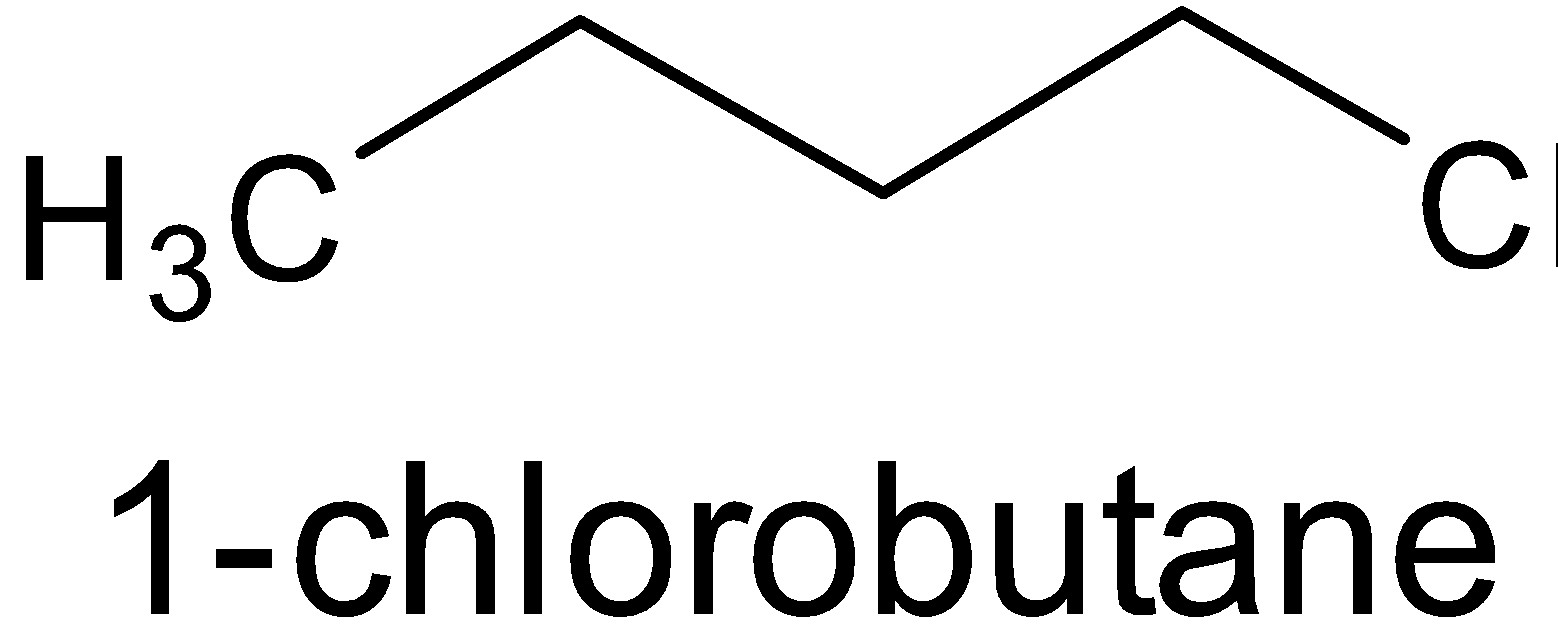 - \[\left( {{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl}}} \right)\]
- \[\left( {{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl}}} \right)\]
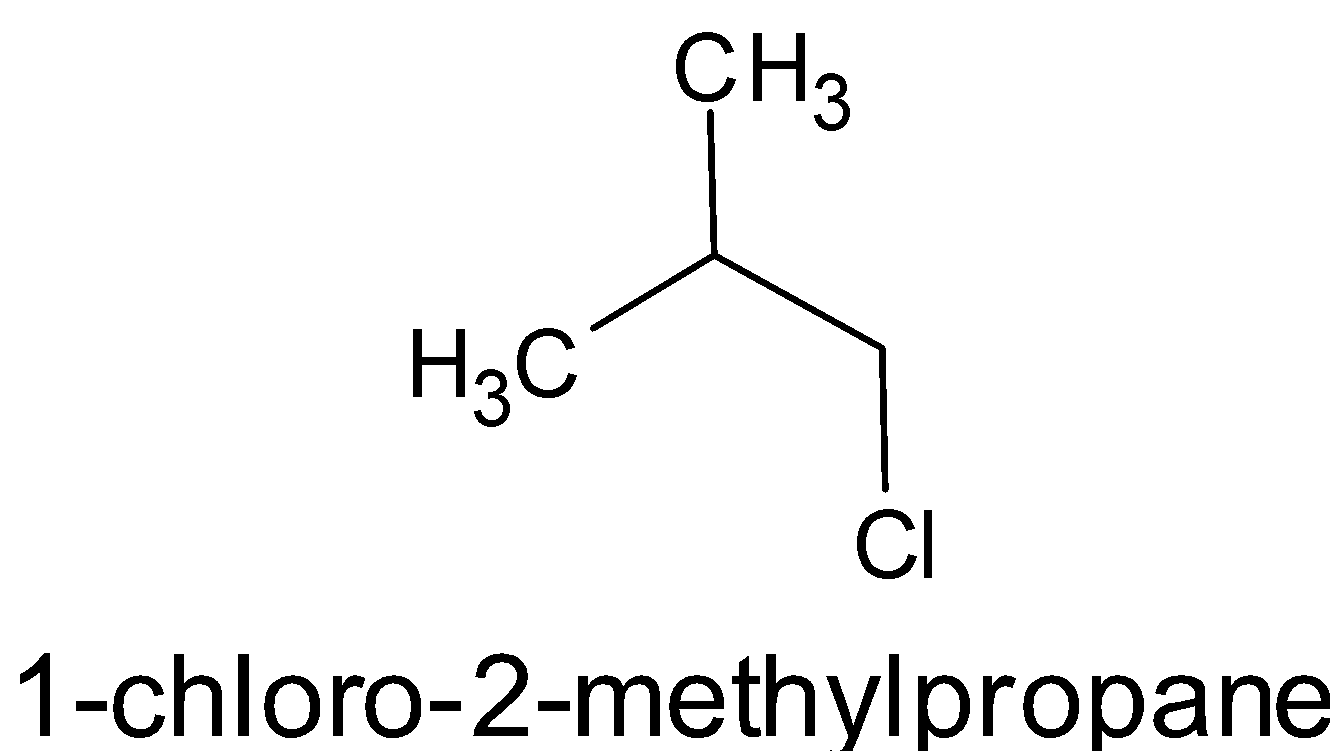 - $\left[ {{{\left( {{{C}}{{{H}}_3}} \right)}_2}{{CHC}}{{{H}}_2}{{Cl}}} \right]$
- $\left[ {{{\left( {{{C}}{{{H}}_3}} \right)}_2}{{CHC}}{{{H}}_2}{{Cl}}} \right]$
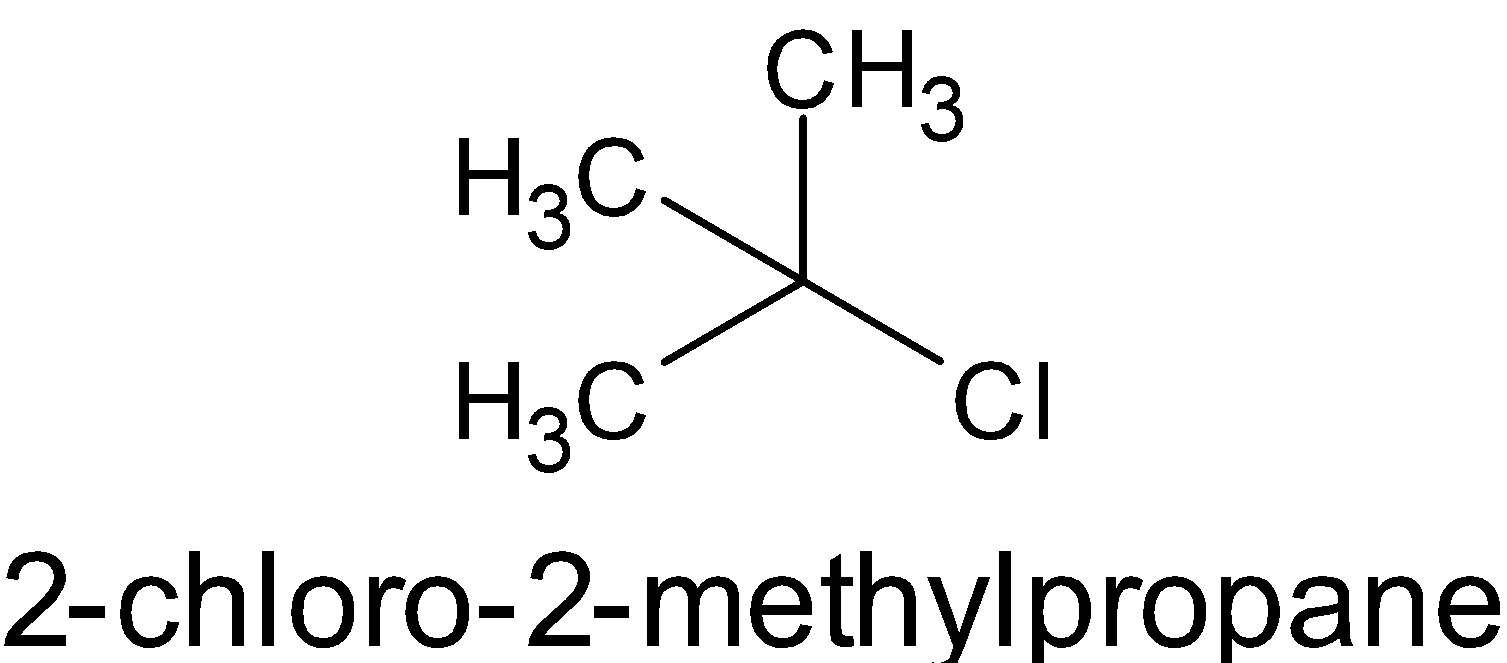 - $\left[ {{{\left( {{{C}}{{{H}}_3}} \right)}_3}{{C}} - {{Cl}}} \right]$
- $\left[ {{{\left( {{{C}}{{{H}}_3}} \right)}_3}{{C}} - {{Cl}}} \right]$
We know that the boiling point is
The boiling point is dependent on the forces between the molecules. Moreover, boiling point increases when the number of carbon atoms is increased. When the number of carbon atoms increases, the surface area and the size of the molecule also increases. There are van der Waals forces between them. This is also increased with the increase in the surface area. When the force is more, more energy, i.e. more temperature is needed for boiling.
As it is obvious that the branched alkanes have less surface area. Thus the force is less. So branched alkanes have less boiling point.
All the compounds are alkyl chlorides. The only difference is that first one is a primary, second one is secondary and the third one is tertiary alkyl halide. From the above structures, it is obvious that the surface area of the third compound is very less than that of the second and first.
So we can express the order of the boiling points of given compounds as:
\[{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl > }}{\left( {{{C}}{{{H}}_3}} \right)_2}{{CHC}}{{{H}}_2}{{Cl}} > {\left( {{{C}}{{{H}}_3}} \right)_3}{{C}} - {{Cl}}\]
Thus $1 - $ chlorobutane has the highest boiling point.
Hence, the correct option is, ‘A. \[{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl}}\]’.
Note: Polarizability is another factor which influences the boiling point. Polar molecules have less boiling points since van der Waals forces in polar molecules are very low. Chlorine has no significant polarizability.
Complete step by step answer:
The structures with their names of the given compounds are given below:



We know that the boiling point is
The boiling point is dependent on the forces between the molecules. Moreover, boiling point increases when the number of carbon atoms is increased. When the number of carbon atoms increases, the surface area and the size of the molecule also increases. There are van der Waals forces between them. This is also increased with the increase in the surface area. When the force is more, more energy, i.e. more temperature is needed for boiling.
As it is obvious that the branched alkanes have less surface area. Thus the force is less. So branched alkanes have less boiling point.
All the compounds are alkyl chlorides. The only difference is that first one is a primary, second one is secondary and the third one is tertiary alkyl halide. From the above structures, it is obvious that the surface area of the third compound is very less than that of the second and first.
So we can express the order of the boiling points of given compounds as:
\[{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl > }}{\left( {{{C}}{{{H}}_3}} \right)_2}{{CHC}}{{{H}}_2}{{Cl}} > {\left( {{{C}}{{{H}}_3}} \right)_3}{{C}} - {{Cl}}\]
Thus $1 - $ chlorobutane has the highest boiling point.
Hence, the correct option is, ‘A. \[{{C}}{{{H}}_3}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{C}}{{{H}}_2}{{Cl}}\]’.
Note: Polarizability is another factor which influences the boiling point. Polar molecules have less boiling points since van der Waals forces in polar molecules are very low. Chlorine has no significant polarizability.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




