
Which among the following detergents is non-ionic?
A. Sodium lauryl sulphate
B. Pentaerythrityl stearate
C. Cetyl trimethyl ammonium chloride
D. Sodium n-dodecyl benzene sulphonate.
Answer
590.4k+ views
Hint: The detergent which does not contain any charge and the head part is hydrophilic are known as non-ionic detergents. Hydrophilic means that the molecule is water-loving and also soluble in the water.
Complete step by step answer:
- In the given question, we have to identify the non-ionic detergent.
- As we know that non-ionic detergent is those detergents which have neither anionic nor cationic charge that means they are neutral due to lack of charge.
- Among the given options, Pentaerythrityl stearate is non - ionic detergent because it does not contain any charge as we can see that the structure of Pentaerythrityl stearate is
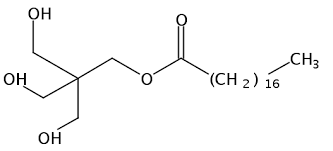
- Also, zwitterionic detergents are a kind of non - ionic detergent because the net charge on them is zero that is the equal amount of both cation and anion charge.
- Now, the first option is an example of Sodium lauryl sulphate which is an example of anionic detergent.
- Anionic detergents are those which consist of the anionic charge at the end of the long chain of sodium salt of sulfonated alcohol or other hydrocarbons. Or we can say that a large part of the chain is made of negative charge.
- The structure of sodium lauryl stearate is:
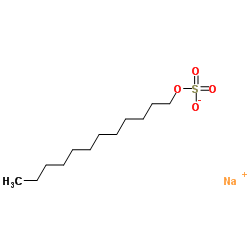
\[\text{N}{{\text{a}}^{+}}\]
- In the third option, Cetyl trimethyl ammonium chloride is given which is an example of cationic detergent.
- Cationic detergent is generally quaternary ammonium salts of chloride, bromide, etc. Also, they have a long chain in which a large part consists of a positive charge.
- The structure of Cetyl trimethyl ammonium chloride is
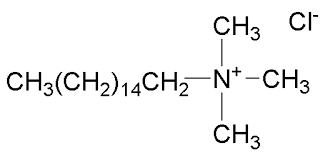
- Now, in the last option, Sodium n-dodecyl benzene sulphonate or SDS is an example of anionic detergent. The structure of the compound is:
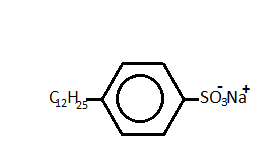
So, the correct answer is “Option B”.
Note: Cationic detergent is widely used in the formation of hair conditioners. Non - ionic detergents are used in the synthesis of liquid dishwashing. Whereas anionic detergents are used in the hand wash and laundry.
Complete step by step answer:
- In the given question, we have to identify the non-ionic detergent.
- As we know that non-ionic detergent is those detergents which have neither anionic nor cationic charge that means they are neutral due to lack of charge.
- Among the given options, Pentaerythrityl stearate is non - ionic detergent because it does not contain any charge as we can see that the structure of Pentaerythrityl stearate is

- Also, zwitterionic detergents are a kind of non - ionic detergent because the net charge on them is zero that is the equal amount of both cation and anion charge.
- Now, the first option is an example of Sodium lauryl sulphate which is an example of anionic detergent.
- Anionic detergents are those which consist of the anionic charge at the end of the long chain of sodium salt of sulfonated alcohol or other hydrocarbons. Or we can say that a large part of the chain is made of negative charge.
- The structure of sodium lauryl stearate is:

\[\text{N}{{\text{a}}^{+}}\]
- In the third option, Cetyl trimethyl ammonium chloride is given which is an example of cationic detergent.
- Cationic detergent is generally quaternary ammonium salts of chloride, bromide, etc. Also, they have a long chain in which a large part consists of a positive charge.
- The structure of Cetyl trimethyl ammonium chloride is

- Now, in the last option, Sodium n-dodecyl benzene sulphonate or SDS is an example of anionic detergent. The structure of the compound is:

So, the correct answer is “Option B”.
Note: Cationic detergent is widely used in the formation of hair conditioners. Non - ionic detergents are used in the synthesis of liquid dishwashing. Whereas anionic detergents are used in the hand wash and laundry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




