
Which among the following are peroxo acids of sulphur?
[A] ${{H}_{2}}S{{O}_{3}}$
[B] ${{H}_{2}}{{S}_{2}}{{O}_{3}}$
[C] ${{H}_{2}}{{S}_{2}}{{O}_{8}}$
[D] ${{H}_{2}}S{{O}_{4}}$
Answer
568.8k+ views
Hint: To answer this, you should know that a peroxo acid is an oxoacid containing a peroxy linkage i.e. an O – O linkage. Draw the structures of the given acids to find the correct answer here. Peroxo acid of sulphur is also known as Marshall’s acid.
Complete Solution :
To answer this question, we have to understand the meaning of peroxo first.
We know that peroxy linkage is an oxygen – oxygen linkage i.e. O – O linkage. Oxo-acids containing these O – O linkage are known as peroxo acids.
Here, we have been asked about peroxo acids of sulphur. Oxo-acids of sulphur with a peroxy linkage are known as peroxo acids of sulphur.
Now, let us go through each of the options to find out the peroxo acids of sulphur among the given options.
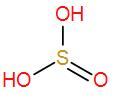
- Firstly we have ${{H}_{2}}S{{O}_{3}}$. It is an inorganic compound and is known as sulphurous acid. We have to draw its structure to find out if it has a peroxy linkage.
We can see it has no O – O linkage therefore it is not the correct answer.
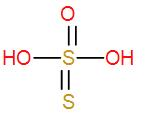
- Then we have ${{H}_{2}}{{S}_{2}}{{O}_{3}}$. It is also an inorganic acid and is known thiosulphuric acid. It is an oxo-acid of sulphur and we can draw its structure as-
It has no O – O linkage therefore it is not a peroxo acid either.
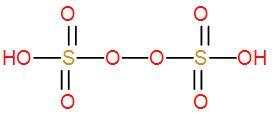
- Next we have ${{H}_{2}}{{S}_{2}}{{O}_{8}}$. It is an inorganic compound and it is known as peroxydisulfuric acid. It is also known as Marshall’s acid. Here the two sulphur atoms are bonded by a peroxy group. We can draw its structure as-
We can see it has an O – O linkage so this is a peroxo acid of sulphur.
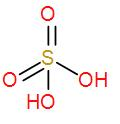
- And lastly we have ${{H}_{2}}S{{O}_{4}}$. We know that it is an inorganic acid and is known as sulphuric acid. We can draw its structure as-
It does not have any O – O linkage so it is not a peroxo acid.
We can understand from the above discussion that peroxydisulfuric acid is a peroxo acid of sulphur.
So, the correct answer is “Option C”.
Note: In peroxydisulfuric acid, sulphur is present in its +6 oxidation state. Both +4 and +6 oxidation states of sulphur are common but +6 oxidation state is strongly acidic and thus is a stronger oxidising agent. Peroxydisulfuric acid is industrially used as a strong oxidising agent and it can be prepared by reaction of chlorosulfonic acid with hydrogen peroxide.
\[2ClS{{O}_{3}}H+{{H}_{2}}{{O}_{2}}\to {{H}_{2}}{{S}_{2}}{{O}_{8}}+2HCl\]
Complete Solution :
To answer this question, we have to understand the meaning of peroxo first.
We know that peroxy linkage is an oxygen – oxygen linkage i.e. O – O linkage. Oxo-acids containing these O – O linkage are known as peroxo acids.
Here, we have been asked about peroxo acids of sulphur. Oxo-acids of sulphur with a peroxy linkage are known as peroxo acids of sulphur.
Now, let us go through each of the options to find out the peroxo acids of sulphur among the given options.
- Firstly we have ${{H}_{2}}S{{O}_{3}}$. It is an inorganic compound and is known as sulphurous acid. We have to draw its structure to find out if it has a peroxy linkage.
We can see it has no O – O linkage therefore it is not the correct answer.

- Then we have ${{H}_{2}}{{S}_{2}}{{O}_{3}}$. It is also an inorganic acid and is known thiosulphuric acid. It is an oxo-acid of sulphur and we can draw its structure as-
It has no O – O linkage therefore it is not a peroxo acid either.

- Next we have ${{H}_{2}}{{S}_{2}}{{O}_{8}}$. It is an inorganic compound and it is known as peroxydisulfuric acid. It is also known as Marshall’s acid. Here the two sulphur atoms are bonded by a peroxy group. We can draw its structure as-
We can see it has an O – O linkage so this is a peroxo acid of sulphur.

- And lastly we have ${{H}_{2}}S{{O}_{4}}$. We know that it is an inorganic acid and is known as sulphuric acid. We can draw its structure as-
It does not have any O – O linkage so it is not a peroxo acid.

We can understand from the above discussion that peroxydisulfuric acid is a peroxo acid of sulphur.
So, the correct answer is “Option C”.
Note: In peroxydisulfuric acid, sulphur is present in its +6 oxidation state. Both +4 and +6 oxidation states of sulphur are common but +6 oxidation state is strongly acidic and thus is a stronger oxidising agent. Peroxydisulfuric acid is industrially used as a strong oxidising agent and it can be prepared by reaction of chlorosulfonic acid with hydrogen peroxide.
\[2ClS{{O}_{3}}H+{{H}_{2}}{{O}_{2}}\to {{H}_{2}}{{S}_{2}}{{O}_{8}}+2HCl\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




