
Which among the following are isostructural?
A.$C{{O}_{2}},{{I}_{3}}^{-}$
B.$Xe{{O}_{2}}{{F}_{2}},S{{F}_{4}}$
C. $S{{O}_{4}}^{2-},N{{O}_{3}}^{-}$
D.$Cl{{F}_{3}},Xe{{F}_{2}}$
Answer
542.1k+ views
Hint: Isostructural compounds are those compounds which have similar structure. Find the shape of the compounds to check whether the compounds are isostructural or not with the help of VSEPR theory. Isoelectronic compounds are also isostructural.
Complete answer:
As you have learnt about the isostructural compounds in your chemistry lessons that this type of compounds have similar structure.
Let us take one of the cases from the given options,
(A). $C{{O}_{2}},{{I}_{3}}^{-}$
-$C{{O}_{2}}$
Carbon dioxide is a covalent compound with 3 atoms, one carbon atom and two oxygen atoms. Carbon has four electrons in their outermost orbital and oxygen has 6 electrons.
So, from here we can say that a carbon atom is attached with two oxygen atoms by forming a double bond between carbon and oxygen. And the hybridization of this compound will be sp showing liner geometry.
So, the structure of will $C{{O}_{2}}$be,
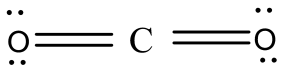
Here, you can see the bond angle between the carbon and oxygen is ${{180}^{o}}$ and the geometry of this compound is linear.
-${{I}_{3}}^{-}$
Triiodide is also a covalent molecule with three iodine molecules. Iodine has seven electrons in their outermost orbital. In this case one iodine atom is attached to two other iodine atoms by sharing their electron and forming a single bond between two iodine atoms.
Here iodine atoms are attached with two bond pairs and three lone pairs surrounding the central atom.
Hybridization of this molecule is $s{{p}^{3}}d$, therefore it should have trigonal bipyramidal geometry but due to the presence of lone pair it gets distorted from its original geometry.
So, the structure of triiodide is,
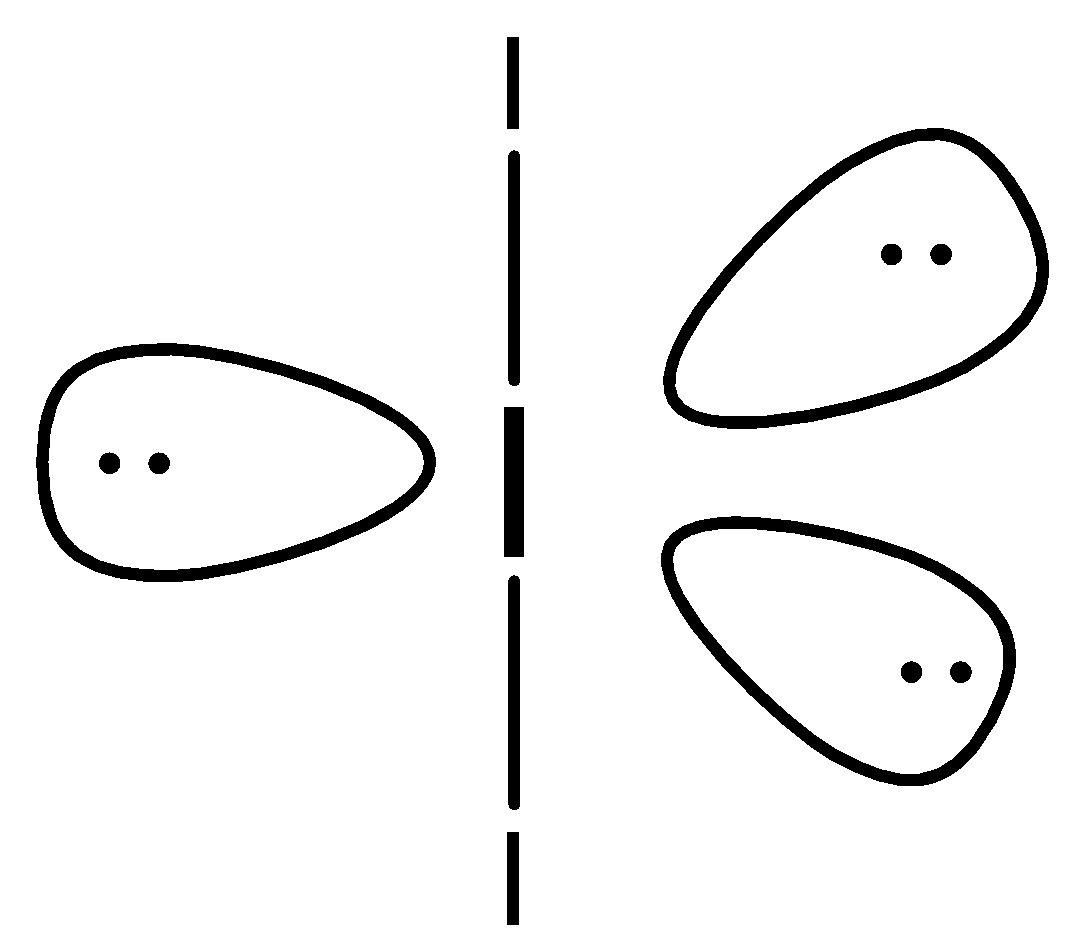
Here, you can see the bond angle between the iodine molecules is ${{180}^{o}}$ and the geometry of this compound is also linear.
So $C{{O}_{2}},{{I}_{3}}^{-}$are isostructural to each other.
But if you will see the second case that is $Xe{{O}_{2}}{{F}_{2}},S{{F}_{4}}$, they are also isostructural because both of them have a seesaw geometry but their hybridization are different. The structures are shown below,

In case of option C), $S{{O}_{4}}^{2-}$has tetrahedral shape based on VSEPR theory but in simple terms it has star shaped geometry and $N{{O}_{3}}^{-}$ has trigonal planar structure as shown below,

In option D), $Cl{{F}_{3}}$ has trigonal bipyramidal shape and $Xe{{F}_{2}}$ has linear shape and lone pair of electrons take the equatorial positions and is as shown below,

Thus the correct options are (A) and (B) both.
Note:
The shapes of the molecules should be similar to be called as an isostructural compound. It is not necessary that hybridization of the compound should also be similar to be an isostructural compound because hybridization can have more than one shape, hybridization can be different.
Complete answer:
As you have learnt about the isostructural compounds in your chemistry lessons that this type of compounds have similar structure.
Let us take one of the cases from the given options,
(A). $C{{O}_{2}},{{I}_{3}}^{-}$
-$C{{O}_{2}}$
Carbon dioxide is a covalent compound with 3 atoms, one carbon atom and two oxygen atoms. Carbon has four electrons in their outermost orbital and oxygen has 6 electrons.
So, from here we can say that a carbon atom is attached with two oxygen atoms by forming a double bond between carbon and oxygen. And the hybridization of this compound will be sp showing liner geometry.
So, the structure of will $C{{O}_{2}}$be,
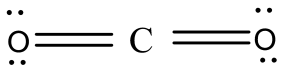
Here, you can see the bond angle between the carbon and oxygen is ${{180}^{o}}$ and the geometry of this compound is linear.
-${{I}_{3}}^{-}$
Triiodide is also a covalent molecule with three iodine molecules. Iodine has seven electrons in their outermost orbital. In this case one iodine atom is attached to two other iodine atoms by sharing their electron and forming a single bond between two iodine atoms.
Here iodine atoms are attached with two bond pairs and three lone pairs surrounding the central atom.
Hybridization of this molecule is $s{{p}^{3}}d$, therefore it should have trigonal bipyramidal geometry but due to the presence of lone pair it gets distorted from its original geometry.
So, the structure of triiodide is,

Here, you can see the bond angle between the iodine molecules is ${{180}^{o}}$ and the geometry of this compound is also linear.
So $C{{O}_{2}},{{I}_{3}}^{-}$are isostructural to each other.
But if you will see the second case that is $Xe{{O}_{2}}{{F}_{2}},S{{F}_{4}}$, they are also isostructural because both of them have a seesaw geometry but their hybridization are different. The structures are shown below,

In case of option C), $S{{O}_{4}}^{2-}$has tetrahedral shape based on VSEPR theory but in simple terms it has star shaped geometry and $N{{O}_{3}}^{-}$ has trigonal planar structure as shown below,

In option D), $Cl{{F}_{3}}$ has trigonal bipyramidal shape and $Xe{{F}_{2}}$ has linear shape and lone pair of electrons take the equatorial positions and is as shown below,

Thus the correct options are (A) and (B) both.
Note:
The shapes of the molecules should be similar to be called as an isostructural compound. It is not necessary that hybridization of the compound should also be similar to be an isostructural compound because hybridization can have more than one shape, hybridization can be different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




