
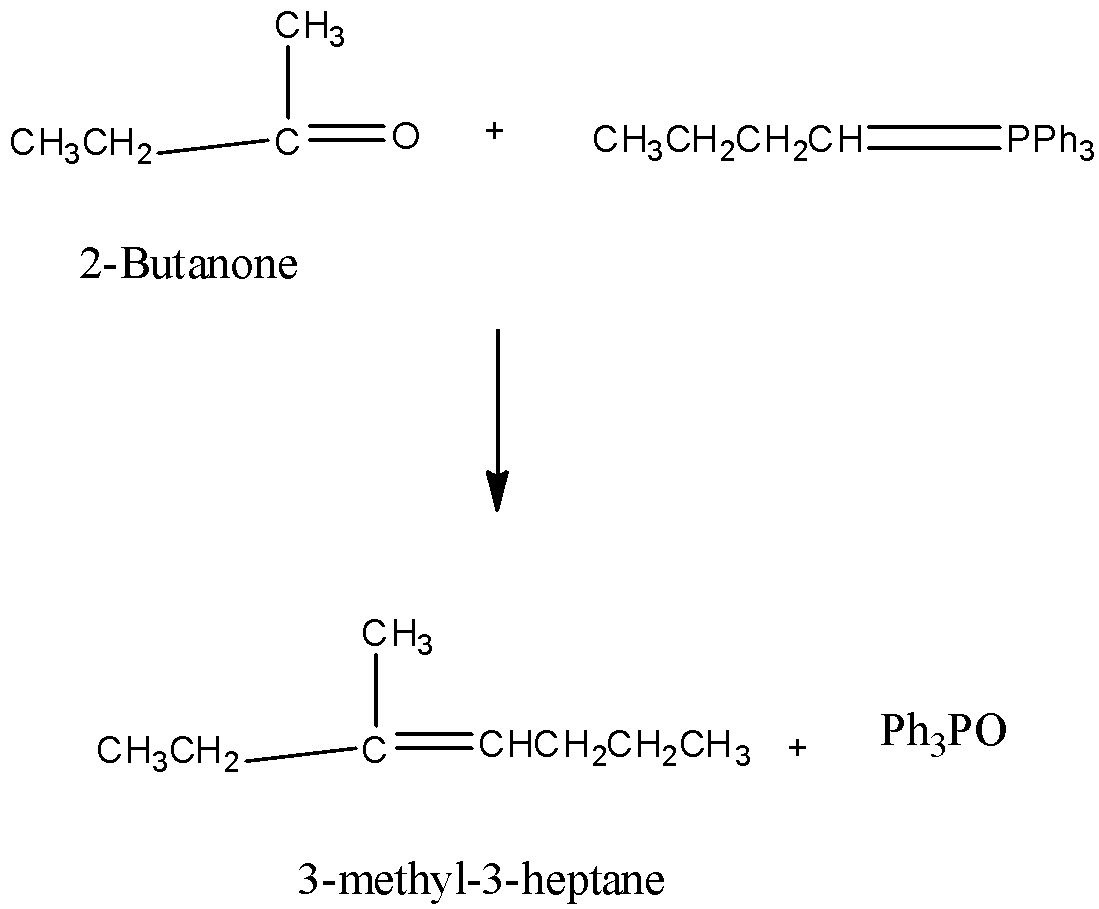
Which alkene is formed from the following reaction \[C{H_{3}}C{H_2}C{H_2}CH = PP{h_3} + 2\] - Butanone ?
A) $3$ -methyl- $3$ -heptane
B) $4$ -methyl- $3$ -heptane
C) $5$ -methyl- $3$ -heptane
D) $1$ -methyl- $5$ -methane
Answer
478.2k+ views
Hint: In the present-day definition given via the means of the IUPAC, a carbocation is any even-electron cation with the huge partial wonderful price on a carbon atom. They are in addition labeled in fundamental classes in step with the coordination variety of the charged carbon: $3$ withinside the carbenium ions and $5$ withinside the carbonium ions.
Complete answer:
• Organolithium or Grignard reagents react with the carbonyl group, \[C = O\] , in aldehydes or ketones to offer alcohols. The substituents at the carbonyl dictate the character of the product alcohol.
• In addition, methanal (formaldehyde) offers the number one alcohol. In addition, different aldehydes offer secondary alcohols. In addition, ketones offer tertiary alcohols.
• The acidic work-up converts an intermediate steel alkoxide salt into the favored alcohol through an easy acid-base response.
• Butanone is a powerful and not unusual place solvent and is utilized in procedures regarding gums, resins, cellulose acetate, and nitrocellulose coatings and in vinyl films.
• The reaction with $2$ -butanone gives $3$ -methyl- $3$ -heptane.
• For this, cause it reveals use withinside the manufacture of plastics, textiles, withinside the manufacturing of paraffin wax, and in family merchandise together with lacquer, varnishes, paint remover, a denaturing agent for denatured alcohol, glues, and as a cleansing agent.
• It has comparable solvent houses to acetone, however it boils at a better temperature and has a considerably slower evaporation rate.
Note:
Unlike acetone, it bureaucracy an azeotrope with water, Butanone, additionally called methyl ethyl ketone (MEK), is a natural compound with the method \[C{H_3}C\left( O \right)C{H_2}C{H_3}\] . This colorless liquid ketone has a sharp, candy smell harking back to acetone. It is produced industrially on a big scale, however happens in nature handiest in hint amounts.
Complete answer:
• Organolithium or Grignard reagents react with the carbonyl group, \[C = O\] , in aldehydes or ketones to offer alcohols. The substituents at the carbonyl dictate the character of the product alcohol.
• In addition, methanal (formaldehyde) offers the number one alcohol. In addition, different aldehydes offer secondary alcohols. In addition, ketones offer tertiary alcohols.
• The acidic work-up converts an intermediate steel alkoxide salt into the favored alcohol through an easy acid-base response.
• Butanone is a powerful and not unusual place solvent and is utilized in procedures regarding gums, resins, cellulose acetate, and nitrocellulose coatings and in vinyl films.
• The reaction with $2$ -butanone gives $3$ -methyl- $3$ -heptane.

• For this, cause it reveals use withinside the manufacture of plastics, textiles, withinside the manufacturing of paraffin wax, and in family merchandise together with lacquer, varnishes, paint remover, a denaturing agent for denatured alcohol, glues, and as a cleansing agent.
• It has comparable solvent houses to acetone, however it boils at a better temperature and has a considerably slower evaporation rate.
Note:
Unlike acetone, it bureaucracy an azeotrope with water, Butanone, additionally called methyl ethyl ketone (MEK), is a natural compound with the method \[C{H_3}C\left( O \right)C{H_2}C{H_3}\] . This colorless liquid ketone has a sharp, candy smell harking back to acetone. It is produced industrially on a big scale, however happens in nature handiest in hint amounts.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




