
Which alkene gives only acetone on ozonolysis?
Answer
524.1k+ views
Hint: Ozonolysis is defined as a reaction in which unsaturated bonds of alkenes, alkynes or azo compounds are broken down with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon – carbon bond is replaced by a carbonyl group.
Complete answer:
To solve this question, let's first understand the mechanism of ozonolysis of alkenes. When alkenes undergo ozonolysis in the presence of zinc and water, the following reaction takes place:
….\[(i)\]
Here, \[R\], \[R'\], , represent the chains of carbon.
The double bonds are broken down by ozone as shown below:
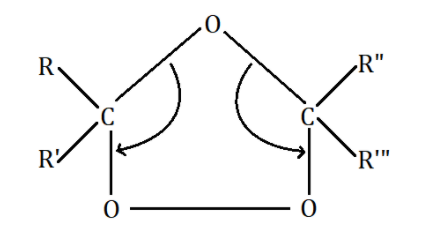
As we can see, the oxygen atom forms bonds with the two carbon atoms where double bonds resided earlier to create an intermediate called ‘Criegee intermediate’. This intermediate decomposes to give us the required carbonyl group i.e., ketones.
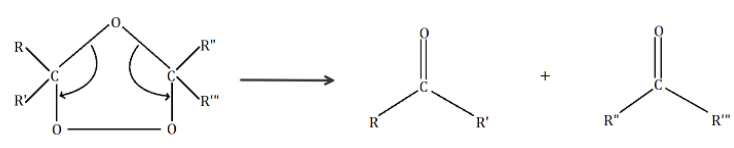
In the given question, we have to identify an alkene compound which on ozonolysis produces acetone only. We know that the formula of acetone is \[{(C{H_3})_2}C = O\] or \[C{H_3}C(O)C{H_3}\].
If we look in the reaction \[(i)\], we have to use a symmetrical alkene to get acetone. Hence, in reaction \[(i)\], we only have to replace \[R\], \[R'\], , with \[C{H_3}\]. On replacing we get \[C{H_3}C(C{H_3}) = C(C{H_3})C{H_3}\] which is \[2,3 - \] Dimethyl \[ - 2 - \] butene. It is a symmetrical alkene.
\[C{H_3}C(C{H_3}) = C(C{H_3})C{H_3} + {O_3}\xrightarrow{{Zn/{H_2}O}}2{(C{H_3})_2}C = O\]
Hence, \[2,3 - \] Dimethyl \[ - 2 - \] butene is the alkene that gives only acetone on ozonolysis.
Note:
Ozonolysis is also used to produce aldehydes. To create aldehydes, alkene compounds with formula \[R - CH = C{H_2}\] are used to produce the aldehydes \[R - CHO\] and \[HCHO\]. Here, \[R\] represents the carbon chain.
In certain reactions of ozonolysis, aldehydes and ketones are both produced. These reactions use unsaturated compounds having formula to give ketones \[R - C(O) - R'\] and aldehydes .
Ozonolysis is considered as an organic redox reaction.
Complete answer:
To solve this question, let's first understand the mechanism of ozonolysis of alkenes. When alkenes undergo ozonolysis in the presence of zinc and water, the following reaction takes place:
….\[(i)\]
Here, \[R\], \[R'\], , represent the chains of carbon.
The double bonds are broken down by ozone as shown below:

As we can see, the oxygen atom forms bonds with the two carbon atoms where double bonds resided earlier to create an intermediate called ‘Criegee intermediate’. This intermediate decomposes to give us the required carbonyl group i.e., ketones.

In the given question, we have to identify an alkene compound which on ozonolysis produces acetone only. We know that the formula of acetone is \[{(C{H_3})_2}C = O\] or \[C{H_3}C(O)C{H_3}\].
If we look in the reaction \[(i)\], we have to use a symmetrical alkene to get acetone. Hence, in reaction \[(i)\], we only have to replace \[R\], \[R'\], , with \[C{H_3}\]. On replacing we get \[C{H_3}C(C{H_3}) = C(C{H_3})C{H_3}\] which is \[2,3 - \] Dimethyl \[ - 2 - \] butene. It is a symmetrical alkene.
\[C{H_3}C(C{H_3}) = C(C{H_3})C{H_3} + {O_3}\xrightarrow{{Zn/{H_2}O}}2{(C{H_3})_2}C = O\]
Hence, \[2,3 - \] Dimethyl \[ - 2 - \] butene is the alkene that gives only acetone on ozonolysis.
Note:
Ozonolysis is also used to produce aldehydes. To create aldehydes, alkene compounds with formula \[R - CH = C{H_2}\] are used to produce the aldehydes \[R - CHO\] and \[HCHO\]. Here, \[R\] represents the carbon chain.
In certain reactions of ozonolysis, aldehydes and ketones are both produced. These reactions use unsaturated compounds having formula to give ketones \[R - C(O) - R'\] and aldehydes .
Ozonolysis is considered as an organic redox reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




