
What would be the products A and B?
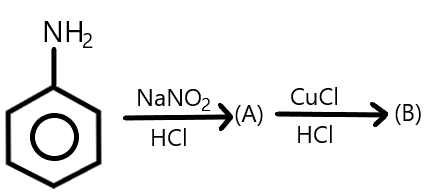

Answer
565.5k+ views
Hint:As we know that primary aromatic amines react with nitrous acid which is formed by the mixture of reaction of sodium nitrite with hydrochloric acid, at low temperatures and results in the formation of aromatic diazonium salts which is commonly called diazotization which further follows Sandmeyer reaction.
Complete answer:As we know that primary aromatic amines react with nitrous acid which is formed by the mixture of reaction of sodium nitrite with hydrochloric acid, at low temperatures such as $273K - 278K$and results in the formation of aromatic diazonium salts which is commonly called diazotization and due to its instability, the diazonium salt is not generally stored and is used immediately after its preparation. We can show this reaction as follows:
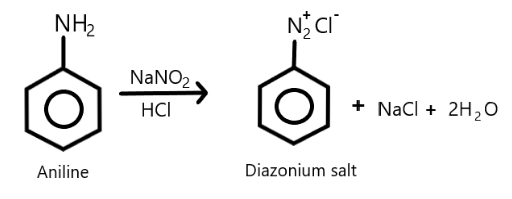
As we know that diazonium salt formed is unstable and we also know that diazonium group is a very good leaving group, so it will be substituted by the other groups such as halogen group where the halide ion basically displaces the nitrogen from the aromatic ring.
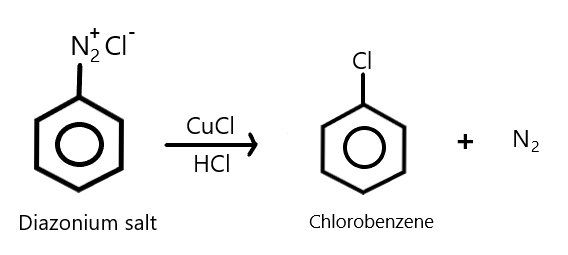
The chloride ion acts as a nucleophile which can easily be introduced in the benzene ring in the presence of copper ion. This reaction is commonly known as Sandmeyer reaction. We can show this using the reaction as:
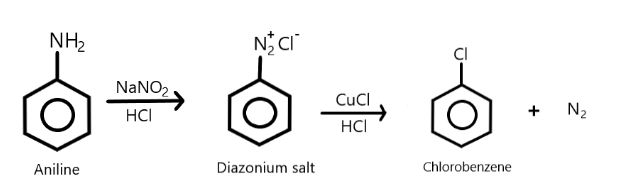
Therefore, from the above explanation we can say that product (A) is Diazonium salt and product (B) is chlorobenzene. We can show this whole reaction as:
Note:Benzenediazonium chloride is a colourless crystalline solid and it is readily soluble in water and is stable in cold water but reacts with water when warmed. It decomposes easily in the dry state. The aryl chloride can also be prepared by Gattermann reaction which involves the treatment of diazonium salt with copper and hydrochloric acid.
Complete answer:As we know that primary aromatic amines react with nitrous acid which is formed by the mixture of reaction of sodium nitrite with hydrochloric acid, at low temperatures such as $273K - 278K$and results in the formation of aromatic diazonium salts which is commonly called diazotization and due to its instability, the diazonium salt is not generally stored and is used immediately after its preparation. We can show this reaction as follows:

As we know that diazonium salt formed is unstable and we also know that diazonium group is a very good leaving group, so it will be substituted by the other groups such as halogen group where the halide ion basically displaces the nitrogen from the aromatic ring.
The chloride ion acts as a nucleophile which can easily be introduced in the benzene ring in the presence of copper ion. This reaction is commonly known as Sandmeyer reaction. We can show this using the reaction as:

Therefore, from the above explanation we can say that product (A) is Diazonium salt and product (B) is chlorobenzene. We can show this whole reaction as:

Note:Benzenediazonium chloride is a colourless crystalline solid and it is readily soluble in water and is stable in cold water but reacts with water when warmed. It decomposes easily in the dry state. The aryl chloride can also be prepared by Gattermann reaction which involves the treatment of diazonium salt with copper and hydrochloric acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




