
What product will be formed?
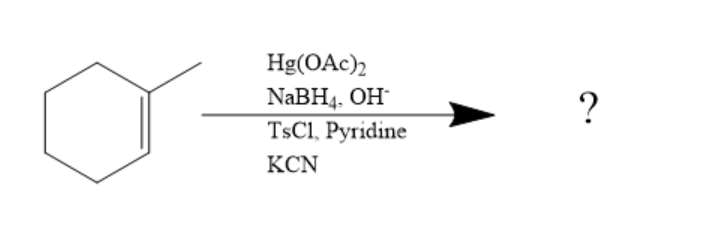


Answer
492.9k+ views
Hint :There are many reagents in organic chemistry for different chemical reactions. $ Hg{(OAc)_2} $ and $ NaB{H_4},O{H^ - } $ when used together they introduce $ - OH $ group on $ s{p^2} $ carbon making it saturated $ s{p^3} $ . $ TsCl $ when attack on the $ - OH $ group converts it into $ - OTs $ which is a very good leaving group and $ KCN $ in the above reaction is used to introduce the $ - CN $ group.
Complete Step By Step Answer:
Reagents are chemical substances which are used in numerous organic reactions to synthesize desired products.
The reaction given is:
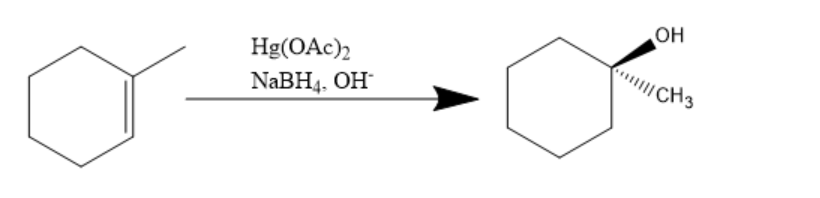
In the above given reaction, first $ Hg{(OAc)_2} $ and $ NaB{H_4},O{H^ - } $ is added and it will introduce $ - OH $ group on $ s{p^2} $ carbon making it saturated $ s{p^3} $ according to markovnikov's rule.
It states that with the addition of HX or other polar reagent to an asymmetric alkene, the hydrogen gets attached to the carbon with more number of hydrogen substituents, and the X group or electronegative part gets attached to the carbon with more number of alkyl substituents.
Hence, the reaction proceeds as:
Now, pyridine (being a base) abstract the hydrogen from hydroxyl group and $ TsCl $ is added, it attack on $ - OH $ group converts it into $ - OTs $ which is a very good leaving group and the reaction proceeds as follow:
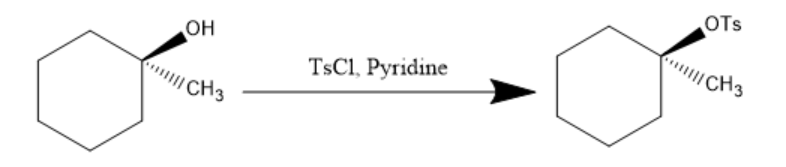
Now, the $ KCN $ is added to the product and $ - OTs $ being a very good leaving group, leaves and $ C{N^ - } $ attacks and is introduced to the compound. The reaction can be written as:
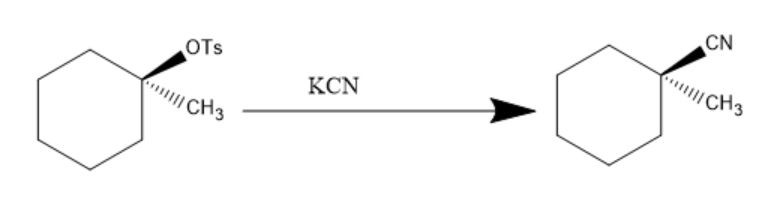
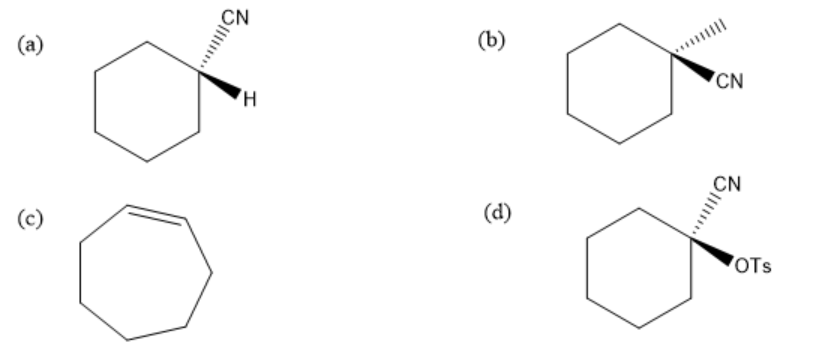
Hence, the final product of above given reaction is:
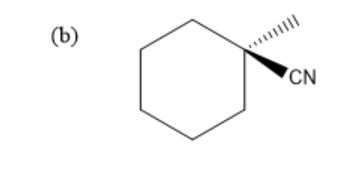
Therefore, the correct option is (b).
Note :
For the addition of hydroxyl group to a double bond (unsaturated bond) through the markovnikov rule, we use reagents as $ B{H_3},THF,{H_2}{O_2} $ and $ OH^{-} $. In this reaction, the hydrogen gets attached to the carbon with more alkyl substituents, and the X group or electronegative part gets attached to the carbon with more hydrogen substituents.
Complete Step By Step Answer:
Reagents are chemical substances which are used in numerous organic reactions to synthesize desired products.
The reaction given is:

In the above given reaction, first $ Hg{(OAc)_2} $ and $ NaB{H_4},O{H^ - } $ is added and it will introduce $ - OH $ group on $ s{p^2} $ carbon making it saturated $ s{p^3} $ according to markovnikov's rule.
It states that with the addition of HX or other polar reagent to an asymmetric alkene, the hydrogen gets attached to the carbon with more number of hydrogen substituents, and the X group or electronegative part gets attached to the carbon with more number of alkyl substituents.
Hence, the reaction proceeds as:

Now, pyridine (being a base) abstract the hydrogen from hydroxyl group and $ TsCl $ is added, it attack on $ - OH $ group converts it into $ - OTs $ which is a very good leaving group and the reaction proceeds as follow:

Now, the $ KCN $ is added to the product and $ - OTs $ being a very good leaving group, leaves and $ C{N^ - } $ attacks and is introduced to the compound. The reaction can be written as:

Hence, the final product of above given reaction is:

Therefore, the correct option is (b).
Note :
For the addition of hydroxyl group to a double bond (unsaturated bond) through the markovnikov rule, we use reagents as $ B{H_3},THF,{H_2}{O_2} $ and $ OH^{-} $. In this reaction, the hydrogen gets attached to the carbon with more alkyl substituents, and the X group or electronegative part gets attached to the carbon with more hydrogen substituents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




