
What makes an amine more basic?
Answer
507k+ views
Hint: Nitrogen of amine is sp3 hybridized. In amine, Nitrogen is bonded to three covalent bonds and consists of one non-bonding pair of electrons. A Lewis base is that substance which has the ability to donate lone pairs of electrons to make covalent bonds.
Complete answer:
Amines are formed when we replace hydrogen atoms from ammonia by an alkyl group. Herein, Nitrogen is sp3 hybridized. Three of the four sp3 hybrid orbitals are involved in the formation of covalent bonds while the fourth one contains a lone pair of electrons. According to Lewis theory, a substance which has the ability to donate lone pairs of electrons to make covalent bonds is called a base. Therefore, amine is a Lewis base and is illustrated as follows.
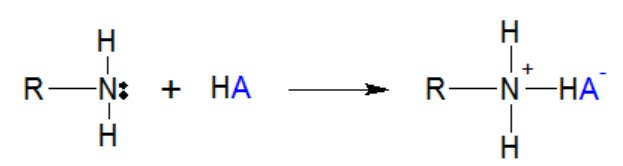
In the above reaction, amine acts as a base and reacts with acid to undergo a neutralization reaction. Amines are of three kinds: Primary, secondary and tertiary amines. In primary amines, N is covalent bonded to one carbon atom while in secondary and tertiary, it is bonded to two and three carbon atoms, respectively. This is illustrate as follows:
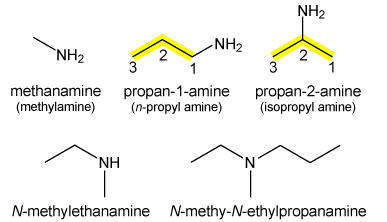
In the above diagram, first row elements describe primary amines while first and second element of second row represent secondary and tertiary amines, respectively. All types of amines contain lone pairs of electrons and differ in the number of alkyl groups. As, alkyl group is electron donating therefore tertiary amine is more basic than secondary which is more basic than primary one.
Therefore, lone pairs of electrons present on nitrogen makes an amine basic in nature. Presence of electron donating group such as alkyl group makes an amine more basic
Note:
It is important to note that lone pairs of electrons present on nitrogen makes an amine basic in nature. Presence of electron donating groups such as alkyl groups makes an amine more basic. More specifically, amines are Lewis bases which have the ability to donate a lone pair of electrons.
Complete answer:
Amines are formed when we replace hydrogen atoms from ammonia by an alkyl group. Herein, Nitrogen is sp3 hybridized. Three of the four sp3 hybrid orbitals are involved in the formation of covalent bonds while the fourth one contains a lone pair of electrons. According to Lewis theory, a substance which has the ability to donate lone pairs of electrons to make covalent bonds is called a base. Therefore, amine is a Lewis base and is illustrated as follows.

In the above reaction, amine acts as a base and reacts with acid to undergo a neutralization reaction. Amines are of three kinds: Primary, secondary and tertiary amines. In primary amines, N is covalent bonded to one carbon atom while in secondary and tertiary, it is bonded to two and three carbon atoms, respectively. This is illustrate as follows:

In the above diagram, first row elements describe primary amines while first and second element of second row represent secondary and tertiary amines, respectively. All types of amines contain lone pairs of electrons and differ in the number of alkyl groups. As, alkyl group is electron donating therefore tertiary amine is more basic than secondary which is more basic than primary one.
Therefore, lone pairs of electrons present on nitrogen makes an amine basic in nature. Presence of electron donating group such as alkyl group makes an amine more basic
Note:
It is important to note that lone pairs of electrons present on nitrogen makes an amine basic in nature. Presence of electron donating groups such as alkyl groups makes an amine more basic. More specifically, amines are Lewis bases which have the ability to donate a lone pair of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




