
What is the vsepr model for \[~N{{H}_{3}}\]?
Answer
533.4k+ views
Hint :We know that Valence shell electron pair repulsion theory, or VSEPR theory, is a concept used in chemistry to determine the structure of single molecules from the number of pairs of electrons surrounding their central atoms.
Complete Step By Step Answer:
Valence-shell electron pair repulsion theory: The Valence Shell Electron Pair Repulsion Theory abbreviated as VSEPR theory is based on the premise that there is a repulsion between the pairs of valence electrons in all atoms, and the atoms will always tend to arrange themselves in a manner in which this electron pair repulsion is minimalized. This arrangement of the atom determines the geometry of the resulting molecule. In a molecule two types of electron pair are there, bond pair and lone pair.
Ammonia is made up of one atom of nitrogen and three atoms of hydrogen. Nitrogen has eight electrons around it i.e. it has four electron pairs. These pairs are arranged tetrahedral. Of the four pairs: one pair is a lone pair. So, the resulting shape is a trigonal pyramid.
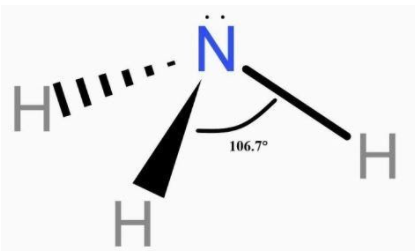
Additional Information:
The repulsion between electron pairs follows the order, lone pair \[\] lone pair \[>\] lone pair \[\] bond pair \[>\] bond pair \[\] bond pair, because different types of repulsion distortion in geometry will be observed from the standard geometry w.r.t hybridization.
Note :
Remember that The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The structure of ammonia molecules as per the valence-shell electron pair repulsion theory is trigonal pyramid.
Complete Step By Step Answer:
Valence-shell electron pair repulsion theory: The Valence Shell Electron Pair Repulsion Theory abbreviated as VSEPR theory is based on the premise that there is a repulsion between the pairs of valence electrons in all atoms, and the atoms will always tend to arrange themselves in a manner in which this electron pair repulsion is minimalized. This arrangement of the atom determines the geometry of the resulting molecule. In a molecule two types of electron pair are there, bond pair and lone pair.
Ammonia is made up of one atom of nitrogen and three atoms of hydrogen. Nitrogen has eight electrons around it i.e. it has four electron pairs. These pairs are arranged tetrahedral. Of the four pairs: one pair is a lone pair. So, the resulting shape is a trigonal pyramid.

Additional Information:
The repulsion between electron pairs follows the order, lone pair \[\] lone pair \[>\] lone pair \[\] bond pair \[>\] bond pair \[\] bond pair, because different types of repulsion distortion in geometry will be observed from the standard geometry w.r.t hybridization.
Note :
Remember that The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The structure of ammonia molecules as per the valence-shell electron pair repulsion theory is trigonal pyramid.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




