
What is the VSEPR model for $ CHC{l_3} $ ?
Answer
531k+ views
Hint :VSEPR is used to predict the shapes of molecules by following a systematic way with the help of numbers of electron pairs to determine the shape of the molecule. And to know how many electrons are there in the outer shell just check it belongs to which group and the group number will be equal to valence electrons.
Complete Step By Step Answer:
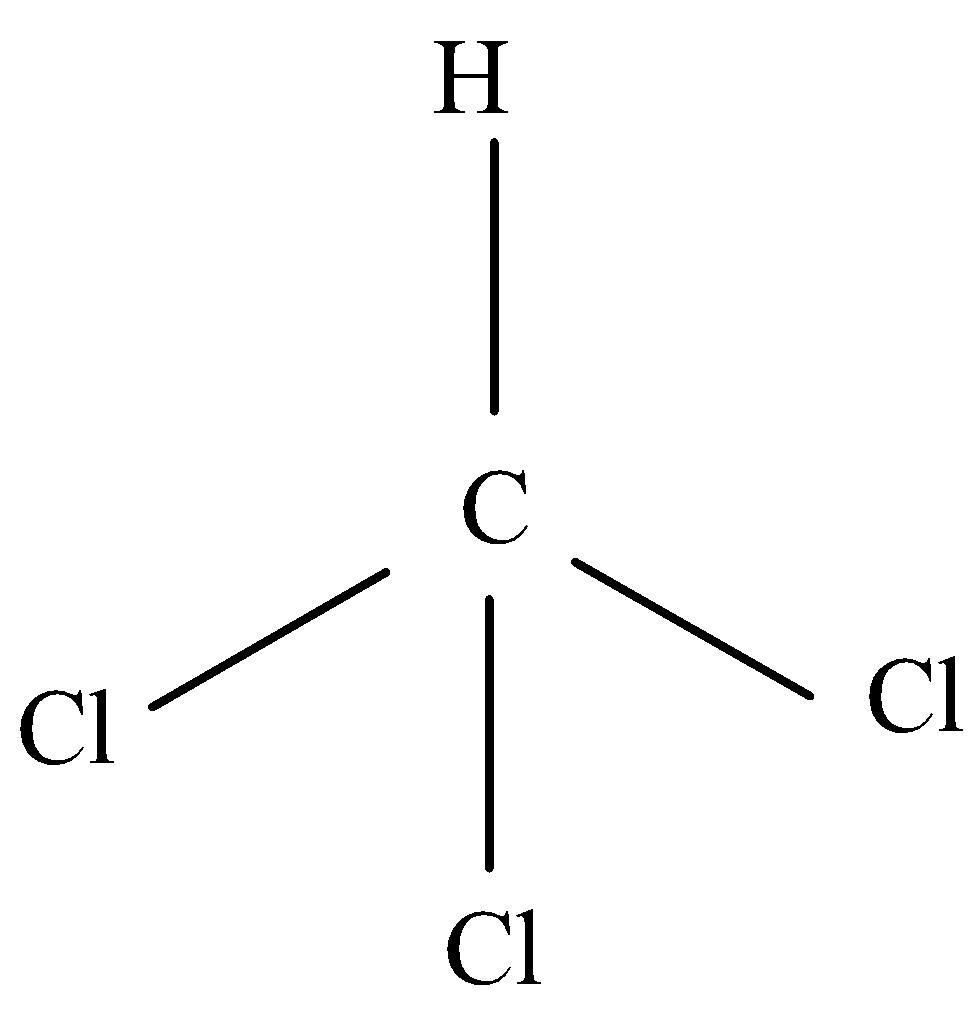
Let's go step by step, first identify the central atom, now the central atom will be $ C. $ Then we will count its valence electrons that are four. Now we will add each electron to each bonding atom. Sometimes we will have to add or subtract electrons for charge. Divide the total of these by two to get the total number of electron pairs and then we will use this number to predict the shape.
There would be three chlorine atoms and a single bond to hydrogen where chlorine will have a single covalent bond. By following all steps, we come to the conclusion that $ CHC{l_3} $ will have $ A{X_4} $ designation. That is, it will make a tetrahedral shape.
Note :
Full form of VSEPR is valence shell electron pair repulsion. It basically states that bonding and nonbonding electron pairs of the central atom in a molecule will push each other away in $ 3D $ space and molecules will have their own shapes.
Complete Step By Step Answer:
Let's go step by step, first identify the central atom, now the central atom will be $ C. $ Then we will count its valence electrons that are four. Now we will add each electron to each bonding atom. Sometimes we will have to add or subtract electrons for charge. Divide the total of these by two to get the total number of electron pairs and then we will use this number to predict the shape.
There would be three chlorine atoms and a single bond to hydrogen where chlorine will have a single covalent bond. By following all steps, we come to the conclusion that $ CHC{l_3} $ will have $ A{X_4} $ designation. That is, it will make a tetrahedral shape.

Note :
Full form of VSEPR is valence shell electron pair repulsion. It basically states that bonding and nonbonding electron pairs of the central atom in a molecule will push each other away in $ 3D $ space and molecules will have their own shapes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




