
What is the 'true boat' conformation?
Answer
491.4k+ views
Hint: Cyclohexane eliminates the eclipsing and angle strain by adopting various non planar conformers. Cyclohexane can form various conformers, each with structural differences, which give different angle strains. The best-known conformers are Chair, Boat, Twist boat and planar
Complete answer:
The chair conformation of cyclohexane is found to be of the lowest energy with a overall ring strain of 0 kJ/mol. The bond angle in the chair conformation was found to be approximately ${111^ \circ }$ which is very near to the tetrahedral bond angle of ${109.28^ \circ }$, hence the angle strain is eliminated. In this conformation the two carbon atoms on the opposite side of the six membered ring are bent out of the plane such that the shape resembles the shape of a reclining beach chair.

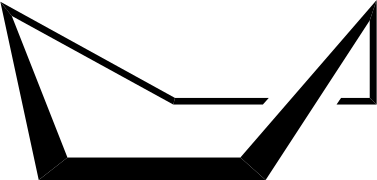
The boat conformation of cyclohexane is formed when the two carbon atoms on the opposite sides of the cyclohexane ring are lifted up out of the plane of the ring creating a shape which resembles a boat. This conformation is less stable than chair because of the steric interactions between 1,4 hydrogens. Because of this it creates a repulsion. The other cause is that the adjacent atoms at the bottom of the boat are forced into an eclipsed position. Hence the boat conformation is less stable than a chair of about 30kJ/mol.
This is the true boat confirmation. The boat conformation is shown below
Note:
The twist boat conformation is more stable than the boat conformer. This conformer reduces the angle strain. The flagpole hydrogen moves farther away and the eight hydrogen become somewhat staggered. The half chair conformation almost has the same energy as the planar cyclohexane. Because of the ${120^ \circ }$ angle, it creates significant angle strain. Half chair is 45 kJ/mol less stable than the chair conformation.
Complete answer:
The chair conformation of cyclohexane is found to be of the lowest energy with a overall ring strain of 0 kJ/mol. The bond angle in the chair conformation was found to be approximately ${111^ \circ }$ which is very near to the tetrahedral bond angle of ${109.28^ \circ }$, hence the angle strain is eliminated. In this conformation the two carbon atoms on the opposite side of the six membered ring are bent out of the plane such that the shape resembles the shape of a reclining beach chair.

The boat conformation of cyclohexane is formed when the two carbon atoms on the opposite sides of the cyclohexane ring are lifted up out of the plane of the ring creating a shape which resembles a boat. This conformation is less stable than chair because of the steric interactions between 1,4 hydrogens. Because of this it creates a repulsion. The other cause is that the adjacent atoms at the bottom of the boat are forced into an eclipsed position. Hence the boat conformation is less stable than a chair of about 30kJ/mol.
This is the true boat confirmation. The boat conformation is shown below

Note:
The twist boat conformation is more stable than the boat conformer. This conformer reduces the angle strain. The flagpole hydrogen moves farther away and the eight hydrogen become somewhat staggered. The half chair conformation almost has the same energy as the planar cyclohexane. Because of the ${120^ \circ }$ angle, it creates significant angle strain. Half chair is 45 kJ/mol less stable than the chair conformation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




