
What is the structure of \[KMn{O_4}\]?
Answer
503.7k+ views
Hint: The chemical formula \[KMn{O_4}\] given in the question is Potassium Permanganate. It is a potassium salts of manganic acid. It is an inorganic versatile chemical compound that appears in bright purple or bronze colour and it has high oxidizing properties.
Complete answer:
The given formula \[KMn{O_4}\] has the chemical name potassium permanganate. It is an inorganic and ionic chemical compound that consists of potassium cation \[({K^ + })\] and permanganate anion \[(Mn{O_4}^ - )\]. In other words, we can say that it is a potassium salts of manganic acid. It is a versatile bright purple or bronze colored compound. It acts as a highly oxidizing agent, a substance that accepts or takes electrons from other substances.
Potassium permanganate is easily soluble in inorganic solvents. It is also soluble in acetone, water, methanol, pyridine and acetic acids.
The crystal structure of \[KMn{O_4}\] is orthorhombic in solid state. Each permanganate anion \[(Mn{O_4}^ - )\] is present in tetrahedral shape.
In permanganate anion \[(Mn{O_4}^ - )\], manganese atom is bonded with four oxygen atoms through three double bonds and one single bond whereas potassium is present as cation \[({K^ + })\]
Structure of potassium permanganate as follows:
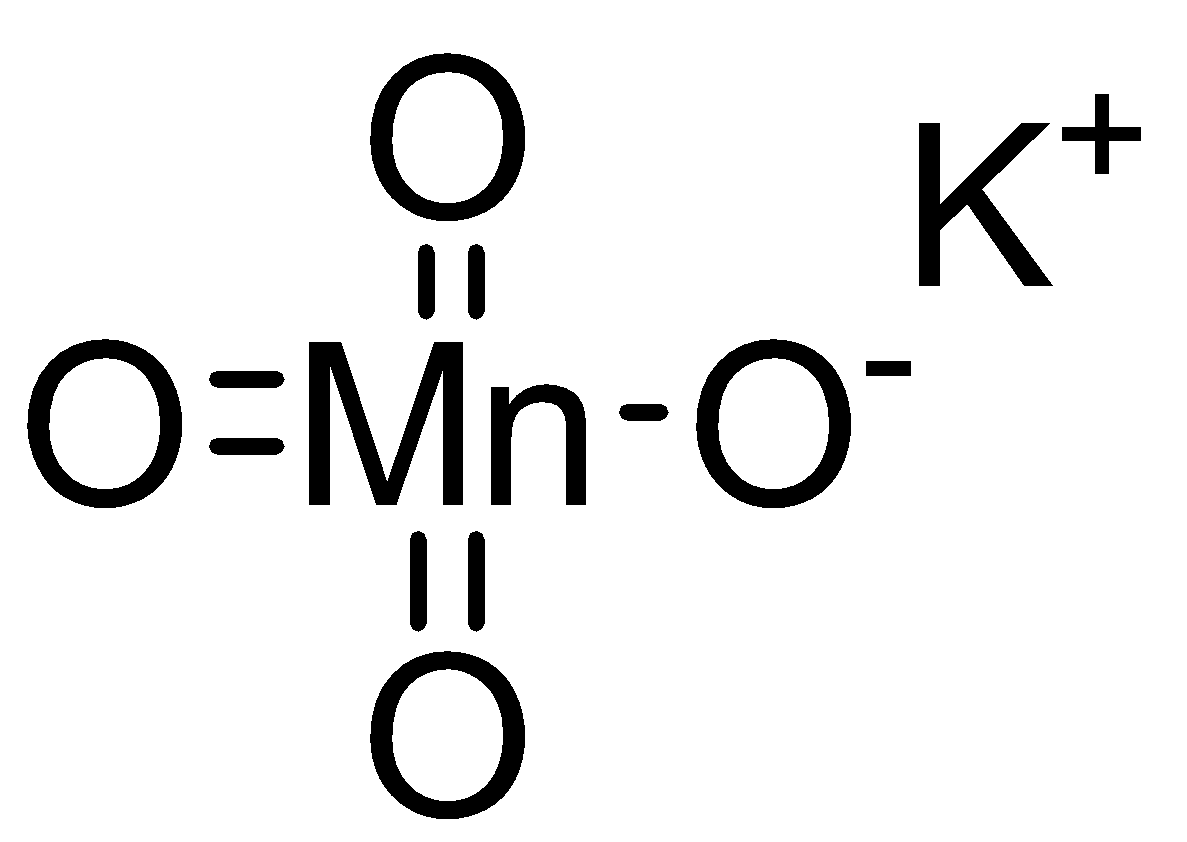
There are many applications of potassium permanganate such as it acts as a disinfectant die to its oxidizing property. It is used to treat skin infections. It is also used in water treatment plants to kill the contaminants and remove foul smell from water. It oxidizes iron, hydrogen sulphide into solid particles which are then removed by filtration. It is also used as a bleaching agent and as a pesticide.
Note:
Besides the wide application of potassium permanganate, it is also a hazardous chemical. Concentrated \[KMn{O_4}\] can cause skin irritation. It can also damage eyes permanently. It also affects the liver and kidneys. Remember, potassium permanganate is also known as “Condy’s crystal or permanganate of potash”
Complete answer:
The given formula \[KMn{O_4}\] has the chemical name potassium permanganate. It is an inorganic and ionic chemical compound that consists of potassium cation \[({K^ + })\] and permanganate anion \[(Mn{O_4}^ - )\]. In other words, we can say that it is a potassium salts of manganic acid. It is a versatile bright purple or bronze colored compound. It acts as a highly oxidizing agent, a substance that accepts or takes electrons from other substances.
Potassium permanganate is easily soluble in inorganic solvents. It is also soluble in acetone, water, methanol, pyridine and acetic acids.
The crystal structure of \[KMn{O_4}\] is orthorhombic in solid state. Each permanganate anion \[(Mn{O_4}^ - )\] is present in tetrahedral shape.
In permanganate anion \[(Mn{O_4}^ - )\], manganese atom is bonded with four oxygen atoms through three double bonds and one single bond whereas potassium is present as cation \[({K^ + })\]
Structure of potassium permanganate as follows:
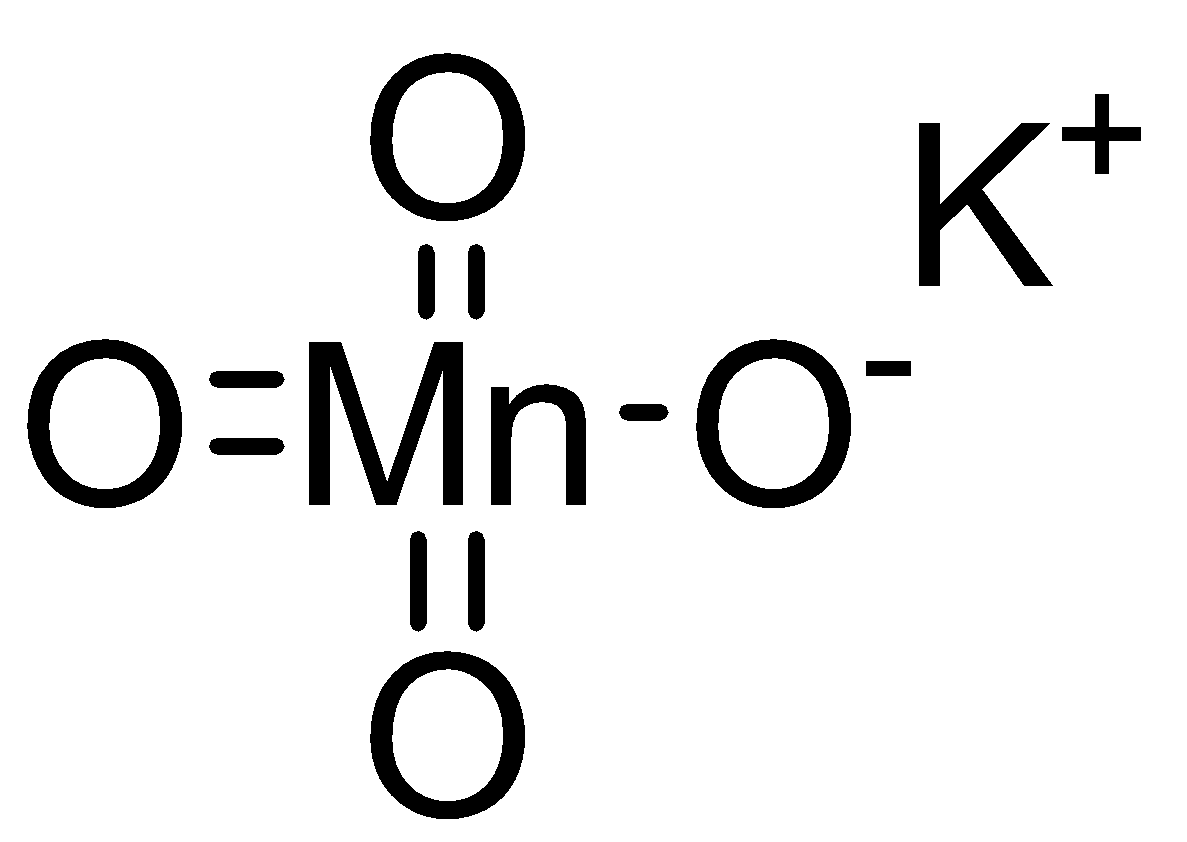
There are many applications of potassium permanganate such as it acts as a disinfectant die to its oxidizing property. It is used to treat skin infections. It is also used in water treatment plants to kill the contaminants and remove foul smell from water. It oxidizes iron, hydrogen sulphide into solid particles which are then removed by filtration. It is also used as a bleaching agent and as a pesticide.
Note:
Besides the wide application of potassium permanganate, it is also a hazardous chemical. Concentrated \[KMn{O_4}\] can cause skin irritation. It can also damage eyes permanently. It also affects the liver and kidneys. Remember, potassium permanganate is also known as “Condy’s crystal or permanganate of potash”
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




