
What is the structure of $ I{F_7} $ ?
Answer
504k+ views
Hint :Iodine hypo fluoride or iodine (VII) chloride are other names for $ I{F_7} $ . It's a strong-bonding interhalogen molecule. According to VSEPR theory, it has a pentagonal bi-pyramidal structure with $ s{p^3}{d^3} $ hybridization.
Complete Step By Step Answer:
The core atom I(iodine) is connected to 7 fluorine atoms via 7 sigma bonds in $ I{F_7} $ . So, in this case, the steric number is 7. As a result, I(iodine) hybridization in $ I{F_7} $ is $ s{p^3}{d^3} $ . As a result, both the electron pair and molecular geometry are pentagonal bi-pyramidal.
Let’s look how the structure comes:
Iodine(atomic no.=53)
$ \left[ {E.C} \right] = 4{d^{10}}5{s^2}5{p^5} $ where E.C=electronic configuration
Look at the following image:
In first excited state its electronic configuration changes:
$ \left[ {E.C} \right] = 5{s^1}5{p^3}5{d^3} $
Hence we can see that there are 7 fluorine molecules. One enters $ 5s $ , 3 can enter $ 5p $ and 3 will enter $ 5d $ .
Hence we can make out that the hybridization is $ s{p^3}{d^3} $ . And hence geometry is pentagonal bi-pyramidal
Let's look at the structure:
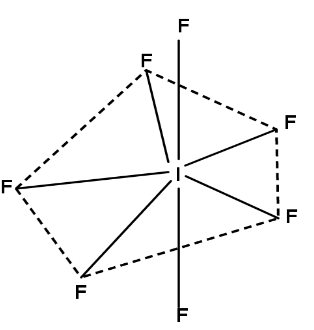
This is pentagonal bi-pyramidal. Also not every bonding angle is the same. Five electron pairs are on the same plane at a 72-degree angle, while the other two are perpendicular to the plane and make a 90-degree angle with it. Even bond lengths differ. The axial bonds are 186pm long, whereas the equatorial bonds are 179pm long.
Note :
There can be many ionization states of an element. But whenever we draw structures we should only consider the first excited state and calculate the hybridization.
Also The primary distinction between axial and equatorial positions is that axial bonds are vertical, whereas equatorial bonds are horizontal. The phrases axial and equatorial are critical when illustrating the real 3D orientation of chemical bonds.
It is not necessary to mention bond angles and bond lengths while drawing the structure.
Complete Step By Step Answer:
The core atom I(iodine) is connected to 7 fluorine atoms via 7 sigma bonds in $ I{F_7} $ . So, in this case, the steric number is 7. As a result, I(iodine) hybridization in $ I{F_7} $ is $ s{p^3}{d^3} $ . As a result, both the electron pair and molecular geometry are pentagonal bi-pyramidal.
Let’s look how the structure comes:
Iodine(atomic no.=53)
$ \left[ {E.C} \right] = 4{d^{10}}5{s^2}5{p^5} $ where E.C=electronic configuration
Look at the following image:
In first excited state its electronic configuration changes:
$ \left[ {E.C} \right] = 5{s^1}5{p^3}5{d^3} $
Hence we can see that there are 7 fluorine molecules. One enters $ 5s $ , 3 can enter $ 5p $ and 3 will enter $ 5d $ .
Hence we can make out that the hybridization is $ s{p^3}{d^3} $ . And hence geometry is pentagonal bi-pyramidal
Let's look at the structure:

This is pentagonal bi-pyramidal. Also not every bonding angle is the same. Five electron pairs are on the same plane at a 72-degree angle, while the other two are perpendicular to the plane and make a 90-degree angle with it. Even bond lengths differ. The axial bonds are 186pm long, whereas the equatorial bonds are 179pm long.
Note :
There can be many ionization states of an element. But whenever we draw structures we should only consider the first excited state and calculate the hybridization.
Also The primary distinction between axial and equatorial positions is that axial bonds are vertical, whereas equatorial bonds are horizontal. The phrases axial and equatorial are critical when illustrating the real 3D orientation of chemical bonds.
It is not necessary to mention bond angles and bond lengths while drawing the structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




