
What is the name of $ NaBr $ ?
Answer
532.2k+ views
Hint :Systematic name, most commonly known as IUPAC name are generally assigned to the compounds. IUPAC stands for International Union of Pure and Applied Chemistry. It is the world authority on the chemical nomenclature as well as terminology. In this question we have to tell the name of $ NaBr $ which is an inorganic compound used widely as a source of bromide ion.
Complete Step By Step Answer:
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure for $ NaBr $ is shown below:
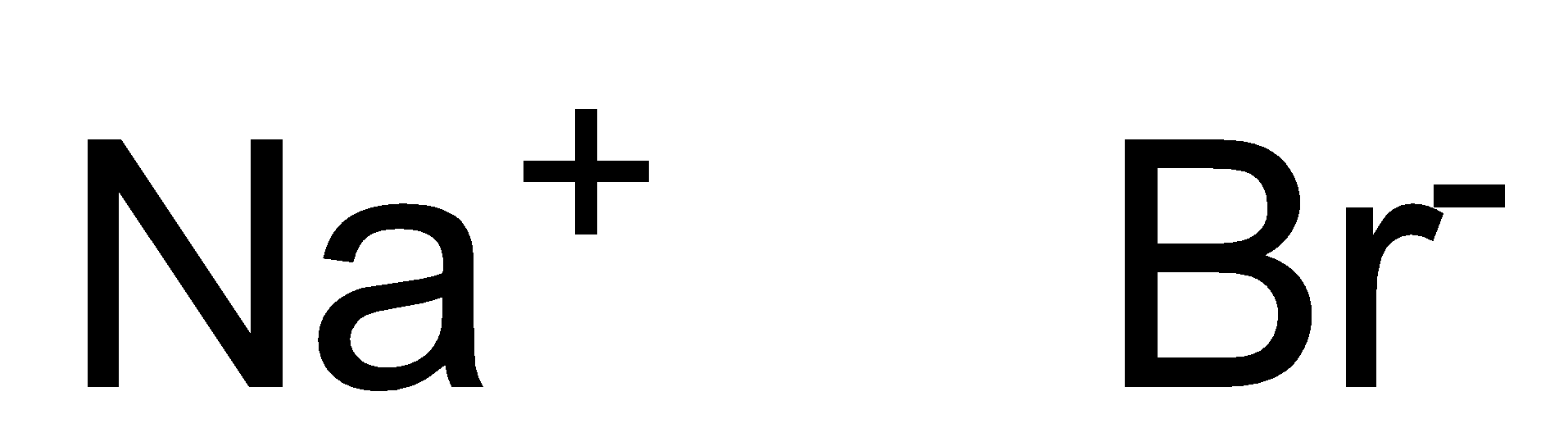
As clear from its chemical formula and structure, $ NaBr $ comprises a metal and a nonmetal. We know that any compound containing a metal and a non-metal is considered to be an ionic compound. Now let us identify the name for the given compound i.e. $ NaBr $ . $ NaBr $ consists of $ Br $ as non-metals after the metal i.e. $ Na $ so we'll use the Periodic Table to identify their names. Rules to write the names of Ionic Compounds are listed below:
1) Name the metal (i.e. cation) according to its appearance in the Periodic Table. For example: $ N{a^ + } = Sodium,{\text{ }}M{g^{2 + }} = Magnesium,{\text{ }}A{l^{3 + }} = Aluminum $
In the present case, $ Na $ stands for sodium.
2) Find the name of the non-metal (i.e. anion) according to its appearance in the Periodic Table and replace its ending with ‘ide’ when you name a compound.
In the present case, $ Br $ stands for bromine and it will be written as bromide.
Hence after combining the name of metal and non-metal, the name of $ NaBr $ is Sodium bromide.
Note :
If you are given the systematic name of an ionic compound and you are asked to find the chemical formula of that compound, take into account the charges of each of the elements present in the compound. Make sure that the charges are balanced such that net charge for the compound must be zero.
Complete Step By Step Answer:
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure for $ NaBr $ is shown below:
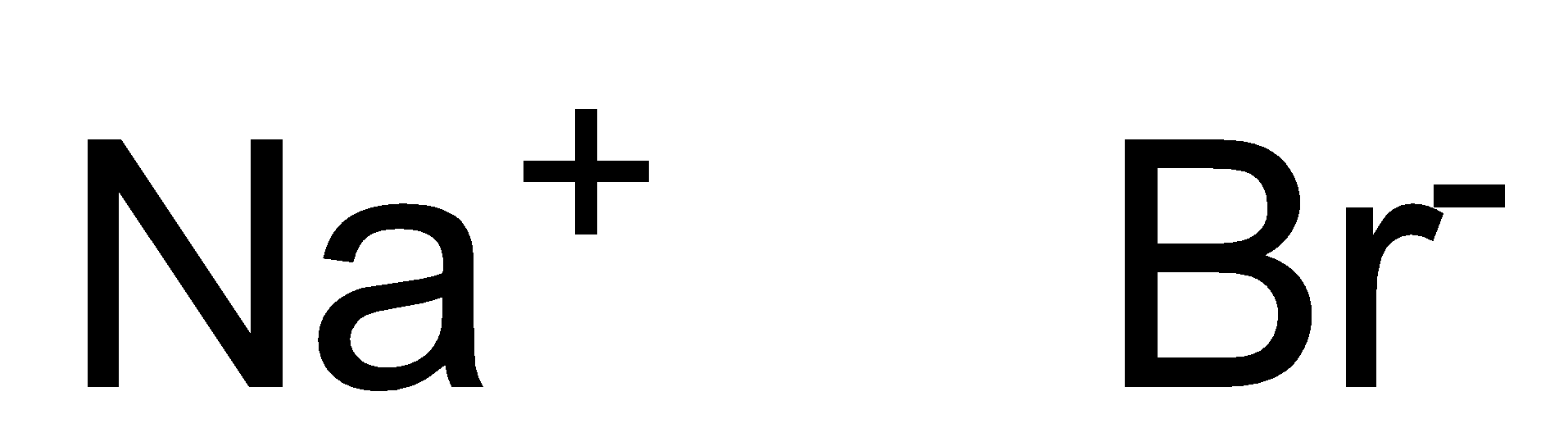
As clear from its chemical formula and structure, $ NaBr $ comprises a metal and a nonmetal. We know that any compound containing a metal and a non-metal is considered to be an ionic compound. Now let us identify the name for the given compound i.e. $ NaBr $ . $ NaBr $ consists of $ Br $ as non-metals after the metal i.e. $ Na $ so we'll use the Periodic Table to identify their names. Rules to write the names of Ionic Compounds are listed below:
1) Name the metal (i.e. cation) according to its appearance in the Periodic Table. For example: $ N{a^ + } = Sodium,{\text{ }}M{g^{2 + }} = Magnesium,{\text{ }}A{l^{3 + }} = Aluminum $
In the present case, $ Na $ stands for sodium.
2) Find the name of the non-metal (i.e. anion) according to its appearance in the Periodic Table and replace its ending with ‘ide’ when you name a compound.
In the present case, $ Br $ stands for bromine and it will be written as bromide.
Hence after combining the name of metal and non-metal, the name of $ NaBr $ is Sodium bromide.
Note :
If you are given the systematic name of an ionic compound and you are asked to find the chemical formula of that compound, take into account the charges of each of the elements present in the compound. Make sure that the charges are balanced such that net charge for the compound must be zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




