
What is the name of ${H_3}P{O_3}$ ?
Answer
581.7k+ views
Hint: In ${H_3}P{O_3}$ , the hydrogen is bonded directly to the central phosphorus atom. It is prepared by dissolving tetraphosphorus hexoxide $({P_4}{O_6})$ or Phosphorous trichloride $(PC{I_3})$ in water.
Complete step by step answer:
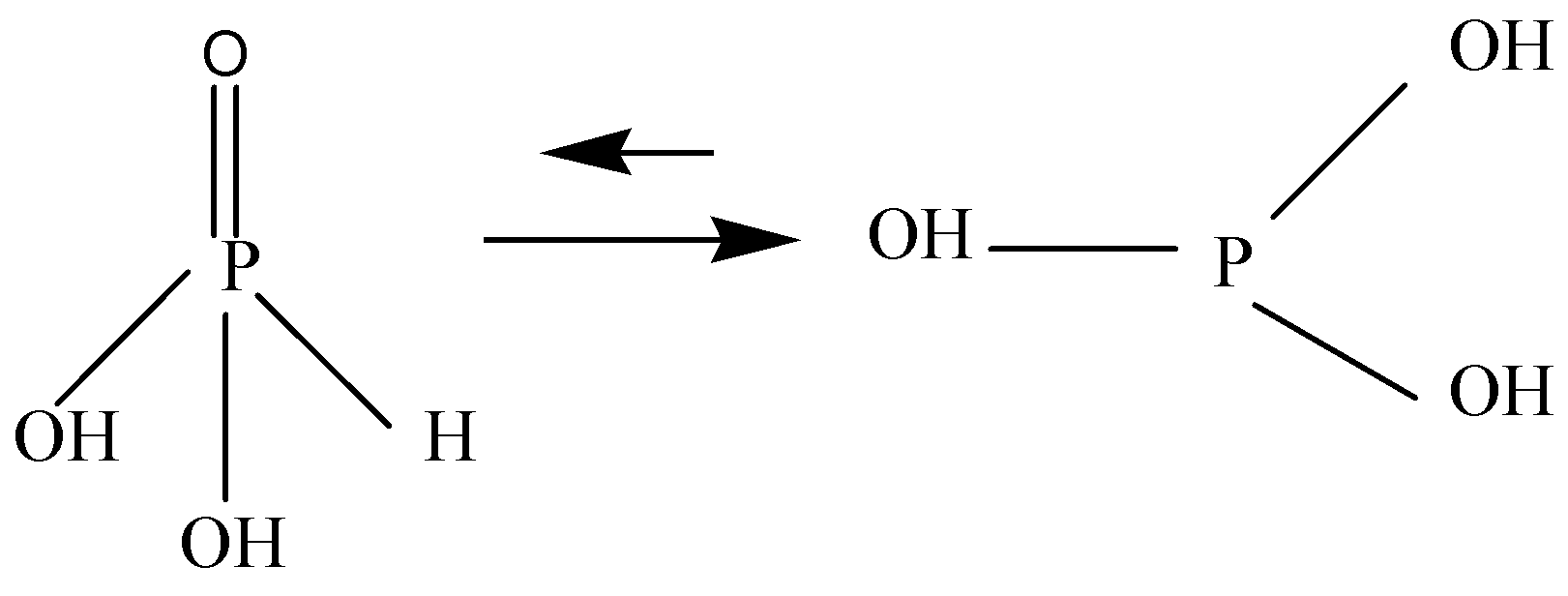
$H_3PO_3$ is ambiguous. It is diprotic and called Phosphorus acid or Orthophosphoric acid, having two hydroxyl groups, one doubly bonded oxygen and one hydrogen atom attached to centrally placed pentavalent phosphorus. It is soluble in water.
Phosphorus acid forms salts called Phosphites, also used as a reducing agent. It is the conjugate acid of Phosphite anion.
It is prepared by dissolving tetraphosphorus hexoxide $({P_4}{O_6})$ or Phosphorous trichloride $(PC{I_3})$ in water. Phosphorous acid is one of several oxygen acids of Phosphorus.
${H_3}P{O_3}$ is not stable and converts into Phosphoric acid due to its strong reducing properties.
Phosphorous acid appears as colorless and odorless crystalline solid. The melting point of Phosphorous acid is \[70.1^\circ C\] or a solution of the solid, its boiling point is $200^\circ C$ and its density is 1.651 \[\dfrac{g}{{c{m^3}}}\] . The contact with Phosphorous acid may severely irritate skin, lips, eyes and mucous membrane which may lead to nausea, vomiting and cramps in the stomach and may result in permanent damage. Phosphoric acid is used in dental cements, as a raw material for synthetic fibers and in the sugar industries.
Note: ${H_3}P{O_3}$ is a conjugate acid of a dihydrogen phosphite. It is a tautomer of phosphonic acid. It has strong reducing properties and tends to be converted into Phosphoric acid. Phosphorous acid is stronger than Phosphoric acid $({H_3}P{O_3})$ .
Complete step by step answer:
$H_3PO_3$ is ambiguous. It is diprotic and called Phosphorus acid or Orthophosphoric acid, having two hydroxyl groups, one doubly bonded oxygen and one hydrogen atom attached to centrally placed pentavalent phosphorus. It is soluble in water.
Phosphorus acid forms salts called Phosphites, also used as a reducing agent. It is the conjugate acid of Phosphite anion.
It is prepared by dissolving tetraphosphorus hexoxide $({P_4}{O_6})$ or Phosphorous trichloride $(PC{I_3})$ in water. Phosphorous acid is one of several oxygen acids of Phosphorus.
${H_3}P{O_3}$ is not stable and converts into Phosphoric acid due to its strong reducing properties.

Phosphorous acid appears as colorless and odorless crystalline solid. The melting point of Phosphorous acid is \[70.1^\circ C\] or a solution of the solid, its boiling point is $200^\circ C$ and its density is 1.651 \[\dfrac{g}{{c{m^3}}}\] . The contact with Phosphorous acid may severely irritate skin, lips, eyes and mucous membrane which may lead to nausea, vomiting and cramps in the stomach and may result in permanent damage. Phosphoric acid is used in dental cements, as a raw material for synthetic fibers and in the sugar industries.
Note: ${H_3}P{O_3}$ is a conjugate acid of a dihydrogen phosphite. It is a tautomer of phosphonic acid. It has strong reducing properties and tends to be converted into Phosphoric acid. Phosphorous acid is stronger than Phosphoric acid $({H_3}P{O_3})$ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




