
What is the name of compound $CO{\text{ ?}}$
Answer
503.7k+ views
Hint: The given compound is formed as the result of incomplete combustion of carbon in the presence of a limited amount of oxygen. The given compound forms a complex with haemoglobin and forms a complex with it and thus even causes the death of a person. This is the by-product of combustion of most fuels.
Complete answer:
When carbon reacts with oxygen, then it is called as combustion of carbon. When this combustion takes place in sufficient amount of oxygen then it forms carbon dioxide which can be shown as,
$C{\text{ + }}{{\text{O}}_2}{\text{ }}\xrightarrow{{}}{\text{ C}}{{\text{O}}_2}$
But when the amount of oxygen is limited then carbon monoxide is formed. This can be represented as,
$C{\text{ + O }}\xrightarrow{{}}{\text{ CO}}$
Here the amount of oxygen is limited. Thus we can say that whenever there is incomplete combustion of carbon and carbon monoxide is formed. Since we know that all fuels are long chains of carbon, thus on incomplete combustion of fuels carbon monoxide is formed as a by-product. The structure of carbon monoxide can be shown as:
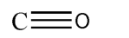
Here the top bond is a co-ordinate bond from oxygen to carbon. While other two bonds formed as a result of mutual sharing between carbon and oxygen. Carbon monoxide is hazardous to living beings as it forms complexes with the blood and causes severe death of living beings.
Note:
Carbon Monoxide is unstable in nature. Therefore it easily forms a complex with the blood of living organisms. Carbon dioxide is more stable than carbon monoxide. It contains coordinate bonds and covalent bonds. It is regarded as toxic gas for living organisms.
Complete answer:
When carbon reacts with oxygen, then it is called as combustion of carbon. When this combustion takes place in sufficient amount of oxygen then it forms carbon dioxide which can be shown as,
$C{\text{ + }}{{\text{O}}_2}{\text{ }}\xrightarrow{{}}{\text{ C}}{{\text{O}}_2}$
But when the amount of oxygen is limited then carbon monoxide is formed. This can be represented as,
$C{\text{ + O }}\xrightarrow{{}}{\text{ CO}}$
Here the amount of oxygen is limited. Thus we can say that whenever there is incomplete combustion of carbon and carbon monoxide is formed. Since we know that all fuels are long chains of carbon, thus on incomplete combustion of fuels carbon monoxide is formed as a by-product. The structure of carbon monoxide can be shown as:
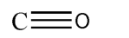
Here the top bond is a co-ordinate bond from oxygen to carbon. While other two bonds formed as a result of mutual sharing between carbon and oxygen. Carbon monoxide is hazardous to living beings as it forms complexes with the blood and causes severe death of living beings.
Note:
Carbon Monoxide is unstable in nature. Therefore it easily forms a complex with the blood of living organisms. Carbon dioxide is more stable than carbon monoxide. It contains coordinate bonds and covalent bonds. It is regarded as toxic gas for living organisms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




