
What is the lone pair effect?
Answer
507k+ views
Hint: Lone pairs in chemistry means a pair of electrons of the valence shell that are not used in bonding purposes with another atom. These are also known as unshared pair of electrons or non-bonding electrons. We can use Lewis structure to find out the lone pair of electrons in an atom. They are usually represented by two dots above the chemical symbol.
Complete answer:
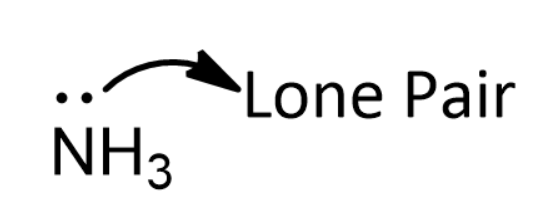
In simple words when the unshared pair of electrons are absolutely shared by another atom, ion or around an atom in the center of the molecule, it is called lone pair effect.
The presence of a lone pair decreases the bond angle between the bonding pairs of atoms. This is due to the high electrical charge within the lone pair that causes great repulsion between the electrons.
This helps in the formation of coordination bonds. We know that Coordination bond occurs when there are one or more lone pairs of electrons that can be donated to the metal orbital such that a bond can be formed.
Note:
Do not confuse this with the inert pair effect. Inert Pair effect is also a very important effect that we have to learn in chemistry. Inert Pair effect is the tendency of s-electrons in the valence shell to remain unshared or not to take part in reactions in compounds that belong to post transition metals.
The d and f orbitals are ineffective in shielding the electrons. Because of this the s-orbital electrons are held more tightly by the nucleus. Thus the inert pair effect increases down the group.

Complete answer:
In simple words when the unshared pair of electrons are absolutely shared by another atom, ion or around an atom in the center of the molecule, it is called lone pair effect.
The presence of a lone pair decreases the bond angle between the bonding pairs of atoms. This is due to the high electrical charge within the lone pair that causes great repulsion between the electrons.
This helps in the formation of coordination bonds. We know that Coordination bond occurs when there are one or more lone pairs of electrons that can be donated to the metal orbital such that a bond can be formed.
Note:
Do not confuse this with the inert pair effect. Inert Pair effect is also a very important effect that we have to learn in chemistry. Inert Pair effect is the tendency of s-electrons in the valence shell to remain unshared or not to take part in reactions in compounds that belong to post transition metals.
The d and f orbitals are ineffective in shielding the electrons. Because of this the s-orbital electrons are held more tightly by the nucleus. Thus the inert pair effect increases down the group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




