
What is the Lewis structure of \[N{H_3}\]?
Answer
506.7k+ views
Hint: We need to know that the ammonia is a \[s{p^3}\] hybridized molecule having trigonal pyramidal geometry. The three hydrogen atoms are attached to the centrally present atom that is nitrogen. Ammonia is a gas having molecular mass $17g/mol$. It is a colorless alkaline gas.
Complete answer:
We need to know that the Lewis structure of ammonia \[N{H_3}\], would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Lewis structure of ammonia can be represented as,
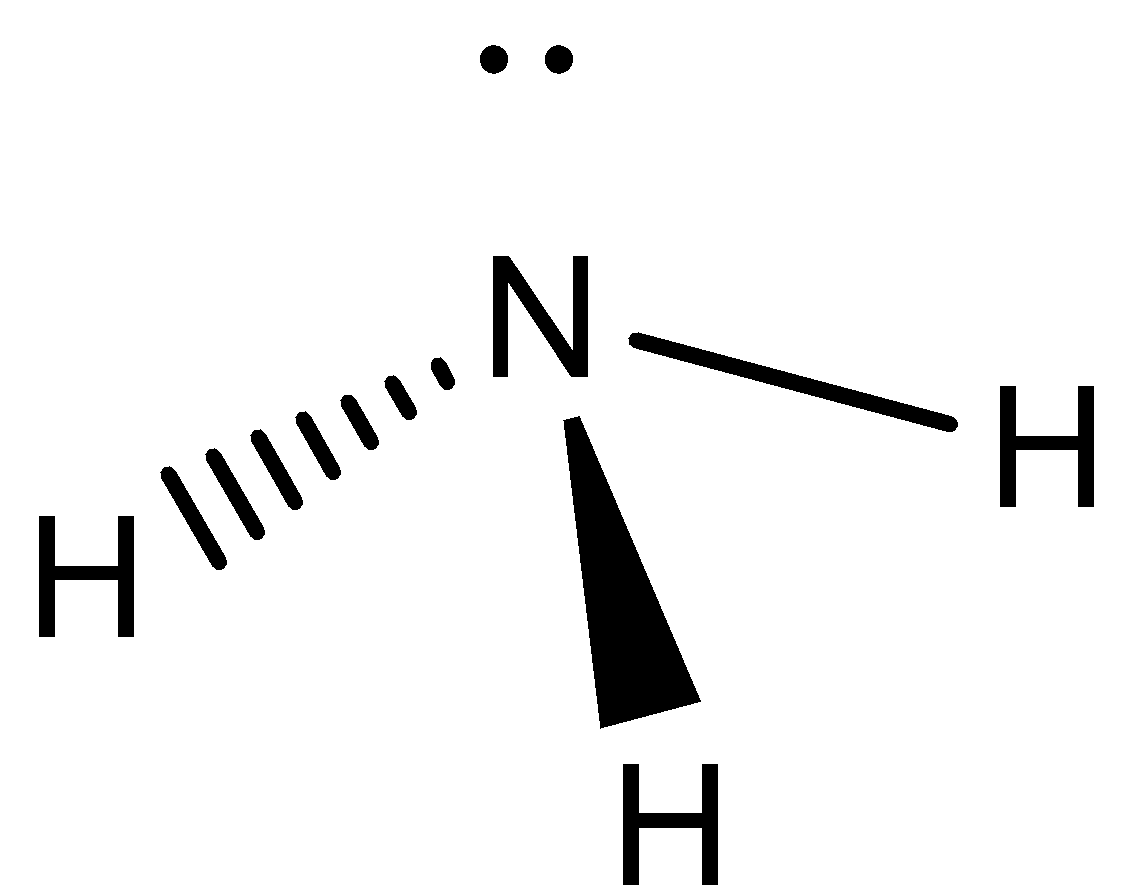
Starting with the Lewis dot structure we know that nitrogen has five valence electrons whereas hydrogen has one valence electron. Thus the total valence electrons will be eight since three hydrogen atoms are present. So, nitrogen is a central atom having three atoms bonded with three hydrogen atoms and left with two valence electrons in total. These two valence electrons will behave as a lone pair of electrons. During hybridization, one s and 3 p orbital form four hybrid orbitals that participate to give the hybridization as \[s{p^3}\]. There are two elements in NH3 hydrogen and nitrogen. Hydrogen is a group IA element and has only one electron in its last shell (valence shell).
Note:
There are two elements in \[N{H_3}\]; hydrogen and nitrogen. Hydrogen is a group \[IA\] element and has only one electron in its last shell (valence shell). Nitrogen is a group \[VA\] element in the periodic table and contains five electrons in its last shell. Nitrogen is a group \[VA\] element in the periodic table and contains five electrons in its last shell.
Complete answer:
We need to know that the Lewis structure of ammonia \[N{H_3}\], would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
Lewis structure of ammonia can be represented as,

Starting with the Lewis dot structure we know that nitrogen has five valence electrons whereas hydrogen has one valence electron. Thus the total valence electrons will be eight since three hydrogen atoms are present. So, nitrogen is a central atom having three atoms bonded with three hydrogen atoms and left with two valence electrons in total. These two valence electrons will behave as a lone pair of electrons. During hybridization, one s and 3 p orbital form four hybrid orbitals that participate to give the hybridization as \[s{p^3}\]. There are two elements in NH3 hydrogen and nitrogen. Hydrogen is a group IA element and has only one electron in its last shell (valence shell).
Note:
There are two elements in \[N{H_3}\]; hydrogen and nitrogen. Hydrogen is a group \[IA\] element and has only one electron in its last shell (valence shell). Nitrogen is a group \[VA\] element in the periodic table and contains five electrons in its last shell. Nitrogen is a group \[VA\] element in the periodic table and contains five electrons in its last shell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




