
What is the lewis structure for $ OC{N^ - } $ ?
Answer
531k+ views
Hint :Lewis structure is also known as lewis dot formulas, it is the diagram that represents valence electrons of atoms within the molecular structure. These structures help to visualise the valence electrons of atoms and to know if they exist as lone pairs or not.
Complete Step By Step Answer:
$ O - C - N $
We will add two double bonds and one triple bond between atoms
$ O = C = N \leftrightarrow O \equiv C - N \leftrightarrow O - C \equiv N $
Now we will show unshared electron pairs around every atom so that an octet of electrons will be shown around it.
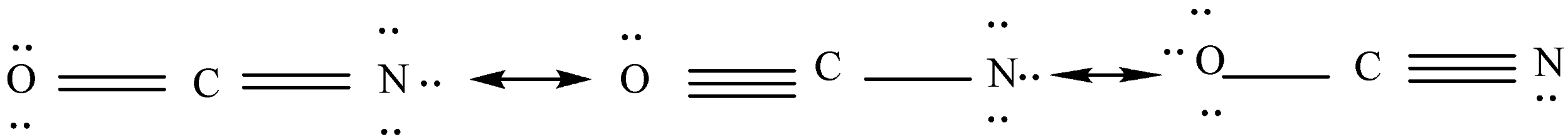
We will calculate the formal charge of each atom according to the last step.
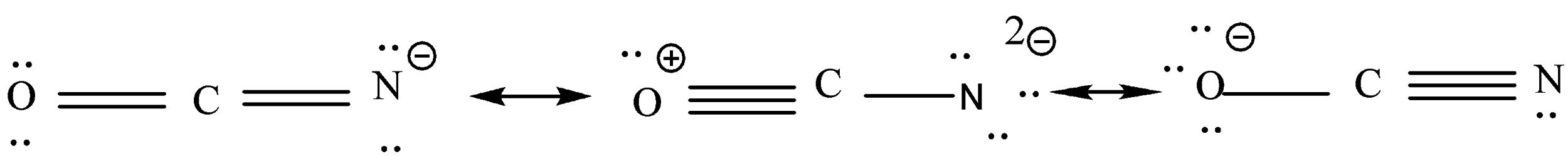
This is resonance structure.
Note :
$ OC{N^ - } $ has a triple bond between carbon and nitrogen atoms and that is why it can be termed as an ambidentate ligand. This cyanate ion is stable. Its electron pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal.
Complete Step By Step Answer:
$ O - C - N $
We will add two double bonds and one triple bond between atoms
$ O = C = N \leftrightarrow O \equiv C - N \leftrightarrow O - C \equiv N $
Now we will show unshared electron pairs around every atom so that an octet of electrons will be shown around it.
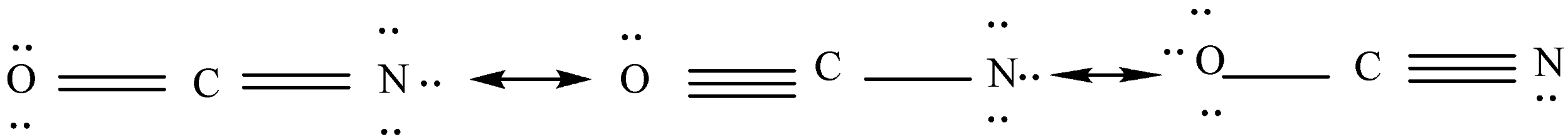
We will calculate the formal charge of each atom according to the last step.
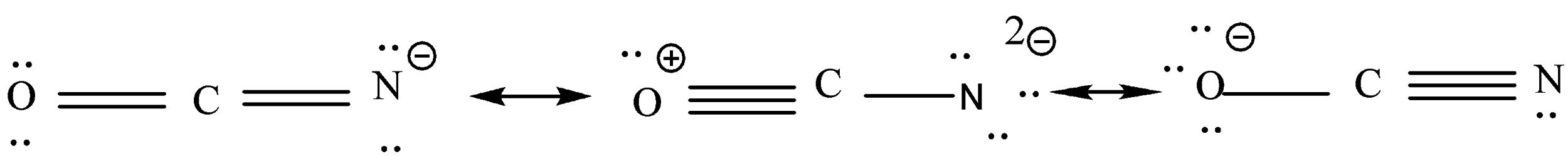
This is resonance structure.
Note :
$ OC{N^ - } $ has a triple bond between carbon and nitrogen atoms and that is why it can be termed as an ambidentate ligand. This cyanate ion is stable. Its electron pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




