
What is the IUPAC nomenclature of
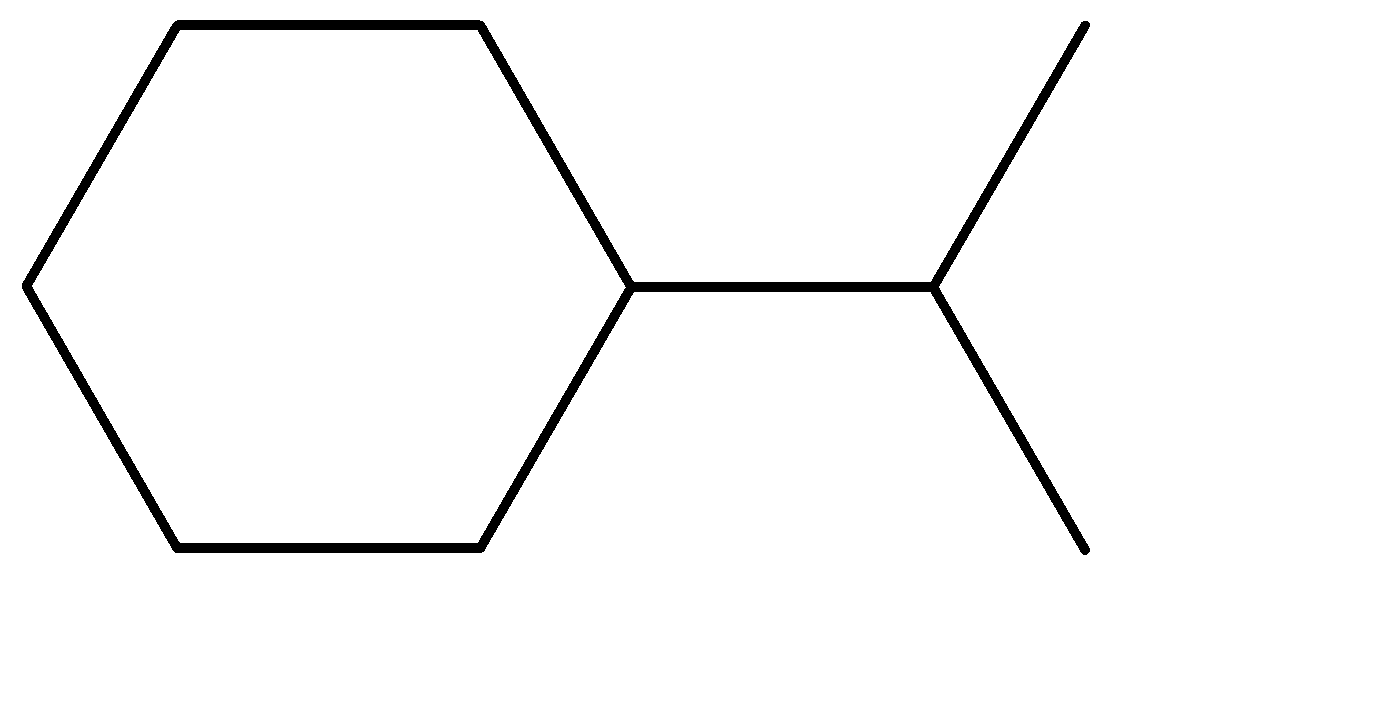

Answer
487.5k+ views
Hint: The given compound to us belongs to the Cyclohexane group. Cyclohexane are the organic molecules which have a six membered closed ring. These compounds are saturated and contain double or triple bonds.
Complete answer:
The compound given to us has a substitute attached to the ring. Since only one substituent is present, we’ll give the substitute the highest priority in numbering. We will first write the IUPAC name of the substituent.
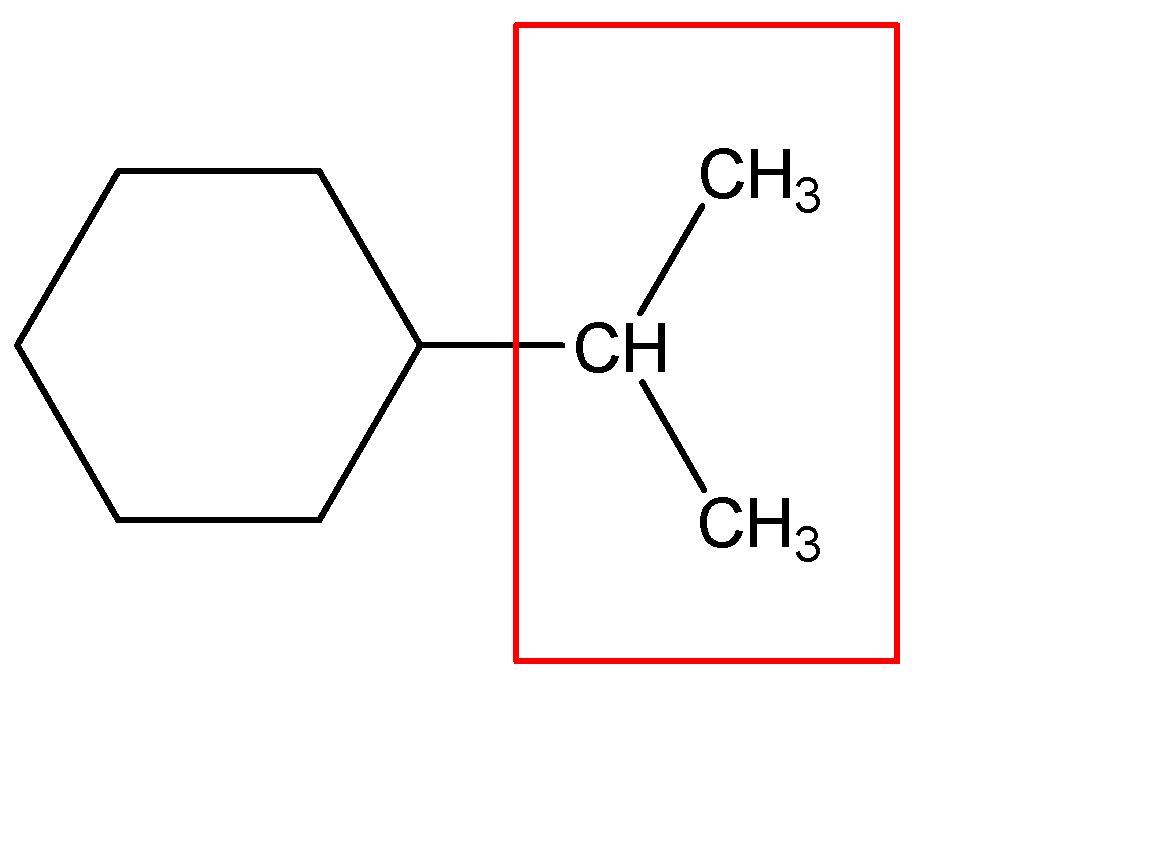
The substituent contains 3 carbons with no double bonds; hence we can call it Propane. But the propane molecule is attached to the cyclohexane ring at the ${C_2}$ position, hence we’ll name it as 2-Propane. If it was an individual molecule, we would have called it 2-Propane itself, but since it is a substituent, the substituent molecule would be Propane-2-yl commonly known as 2-Propyl.
The parent chain/compound is Cyclohexane. Adding the substituent as a prefix to the parent compound, we get the final IUPAC nomenclature as Propane-2-yl cyclohexane. This is the required answer.
Note:
Always remember to give the substituent the first priority, if more than one substituent are present the nomenclature would be done alphabetically. The substituent should be given the minimum number during the numbering of the carbon atoms in the parent chain.
If more than one identical substituents are present the prefixes di, tri, tetra are used. Always consider the longest chain as the parent chain in case of Aliphatic Compounds. In case of aromatic and cyclo compounds, the ring will always be the parent compound.
Complete answer:
The compound given to us has a substitute attached to the ring. Since only one substituent is present, we’ll give the substitute the highest priority in numbering. We will first write the IUPAC name of the substituent.

The substituent contains 3 carbons with no double bonds; hence we can call it Propane. But the propane molecule is attached to the cyclohexane ring at the ${C_2}$ position, hence we’ll name it as 2-Propane. If it was an individual molecule, we would have called it 2-Propane itself, but since it is a substituent, the substituent molecule would be Propane-2-yl commonly known as 2-Propyl.
The parent chain/compound is Cyclohexane. Adding the substituent as a prefix to the parent compound, we get the final IUPAC nomenclature as Propane-2-yl cyclohexane. This is the required answer.
Note:
Always remember to give the substituent the first priority, if more than one substituent are present the nomenclature would be done alphabetically. The substituent should be given the minimum number during the numbering of the carbon atoms in the parent chain.
If more than one identical substituents are present the prefixes di, tri, tetra are used. Always consider the longest chain as the parent chain in case of Aliphatic Compounds. In case of aromatic and cyclo compounds, the ring will always be the parent compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




