
What is the IUPAC name of
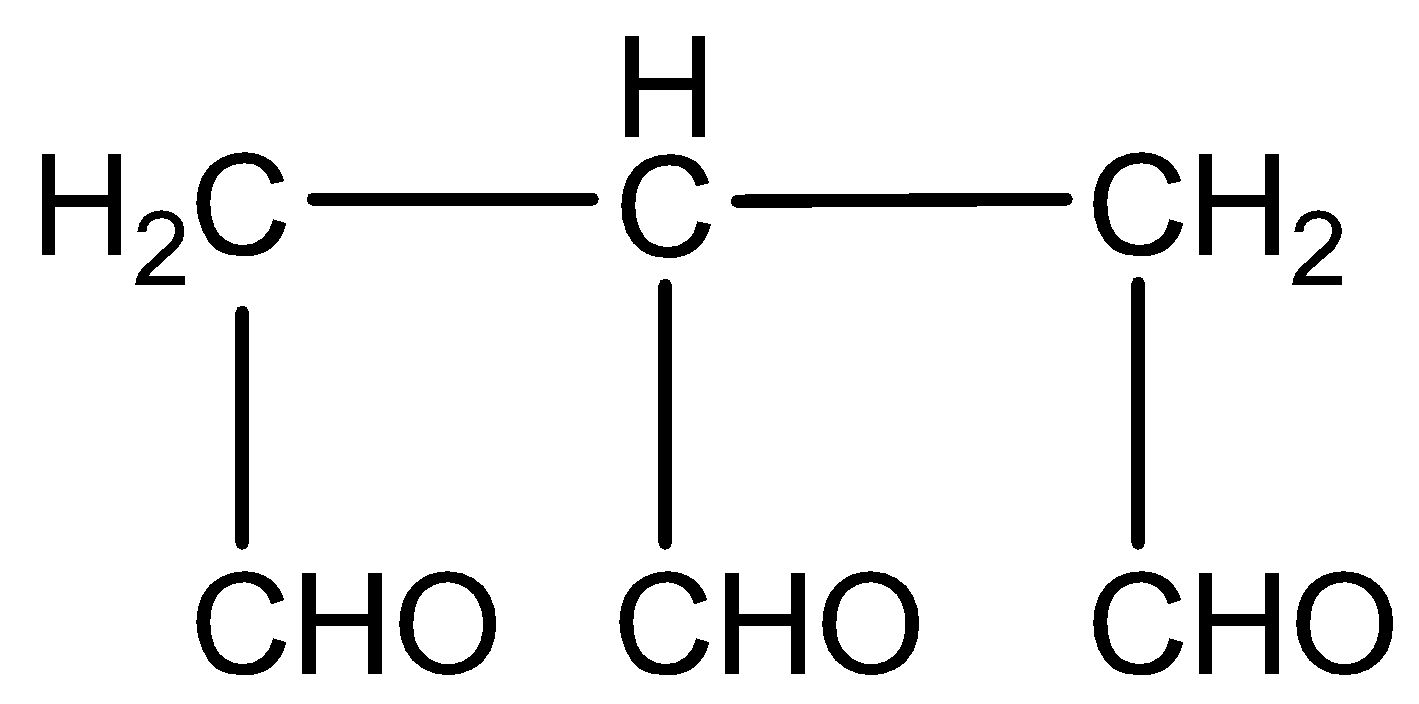

Answer
576.9k+ views
Hint: The parent hydrocarbon contains three Carbon atoms and is called propane. It contains three aldehyde groups \[ - CHO\]. And carbaldehyde is the name given to the CHO group when it is attached to another entity.
Complete step by step answer:
The structure of the above compound is
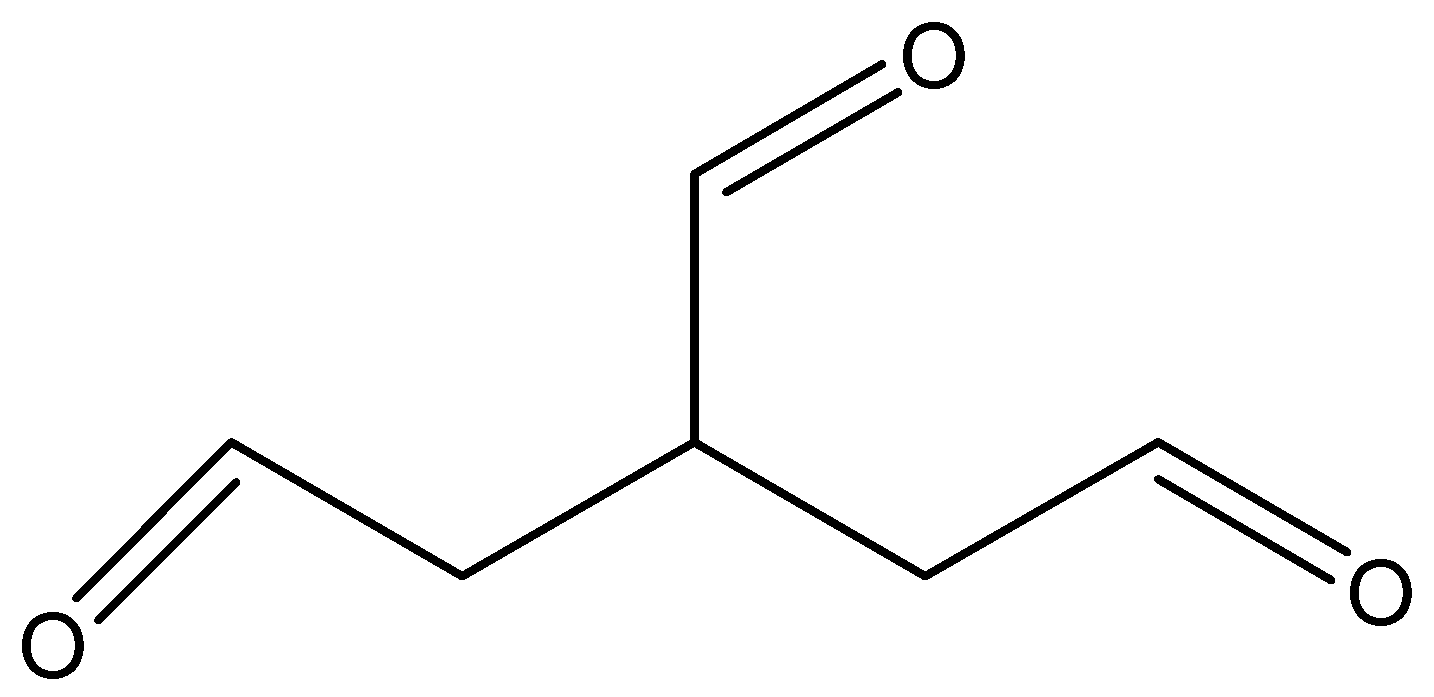
And its molecular formula is ${C_6}{H_8}{O_3}$.
This chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together.
There are three carbon atoms, so we will use ‘Propene’. We see that three \[ - CHO\] groups are attached to the three carbon atoms and we use 'carbaldehyde' as the suffix when the \[ - CHO\] group is attached to a chain. We will give equal preference to all three aldehyde groups. And in this structure, 3 aldehydic groups are attached to 1, 2, 3 positions of propane.
So, its name will be propane-1,2,3-tricarbaldehyde.
The propane-1,2,3-tricarbaldehyde molecule contains a total of 16 bonds. There are 8 non-H bonds, 3 multiple bonds, 5 rotatable bonds, 3 double bonds and 3 aldehydes.
The molecular weight of ${C_6}{H_8}{O_3}$ is 128.12592 \[\dfrac{g}{{mole}}\].
Therefore, the correct IUPAC name is propane-1,2,3-tricarbaldehyde.
Note: The simplest hydrocarbons with all \[C - C\] bonds are alkanes. That is the reason they are called saturated hydrocarbons. The general formula for alkanes is ${C_n}{H_{2n + 2}}$. In alkanes. all carbon atoms tend to complete their tetra valency by bonding with the same or different atoms and all the carbon atoms form single covalent bonds with other carbon atoms. The parent chain can be branched or unbranched and on the basis of that chemical ${C_n}{H_{2n + 2}}$ and physical properties change. Alkanes are comparatively less reactive than hydrocarbons like alkenes, alkynes etc. because all carbon atoms are bonded with single covalent bonds in alkanes which are strong and less reactive in comparison to double or triple covalent bonds of alkenes and alkynes respectively.
Complete step by step answer:
The structure of the above compound is

And its molecular formula is ${C_6}{H_8}{O_3}$.
This chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together.
There are three carbon atoms, so we will use ‘Propene’. We see that three \[ - CHO\] groups are attached to the three carbon atoms and we use 'carbaldehyde' as the suffix when the \[ - CHO\] group is attached to a chain. We will give equal preference to all three aldehyde groups. And in this structure, 3 aldehydic groups are attached to 1, 2, 3 positions of propane.
So, its name will be propane-1,2,3-tricarbaldehyde.
The propane-1,2,3-tricarbaldehyde molecule contains a total of 16 bonds. There are 8 non-H bonds, 3 multiple bonds, 5 rotatable bonds, 3 double bonds and 3 aldehydes.
The molecular weight of ${C_6}{H_8}{O_3}$ is 128.12592 \[\dfrac{g}{{mole}}\].
Therefore, the correct IUPAC name is propane-1,2,3-tricarbaldehyde.
Note: The simplest hydrocarbons with all \[C - C\] bonds are alkanes. That is the reason they are called saturated hydrocarbons. The general formula for alkanes is ${C_n}{H_{2n + 2}}$. In alkanes. all carbon atoms tend to complete their tetra valency by bonding with the same or different atoms and all the carbon atoms form single covalent bonds with other carbon atoms. The parent chain can be branched or unbranched and on the basis of that chemical ${C_n}{H_{2n + 2}}$ and physical properties change. Alkanes are comparatively less reactive than hydrocarbons like alkenes, alkynes etc. because all carbon atoms are bonded with single covalent bonds in alkanes which are strong and less reactive in comparison to double or triple covalent bonds of alkenes and alkynes respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




