
What is the formula of sulfurous acid?
Answer
504k+ views
Hint: We have to know that, sulfurous corrosive is a destructive, non-ignitable, and harmful compound. Ingesting, or breathing in or skin contact with the compound arrangement causes an extreme physical issue, prompting passing. Sulfurous corrosive, in its liquid structure, can seriously consume skin and eyes.
Complete answer:
We have to know that the sulfurous or sulfurous corrosive recipe ought not be mistaken for the equation of sulphuric corrosiveness. While the equation may appear to be comparable yet one of the principle contrasts is that there is less number of oxygen particles in sulfur corrosive. Their physical and synthetic properties additionally contrast partly. Sulfurous corrosive is commonly a dibasic or feeble corrosive and is a watery arrangement of water and sulfur dioxide. It is dry and has a sharp scent.
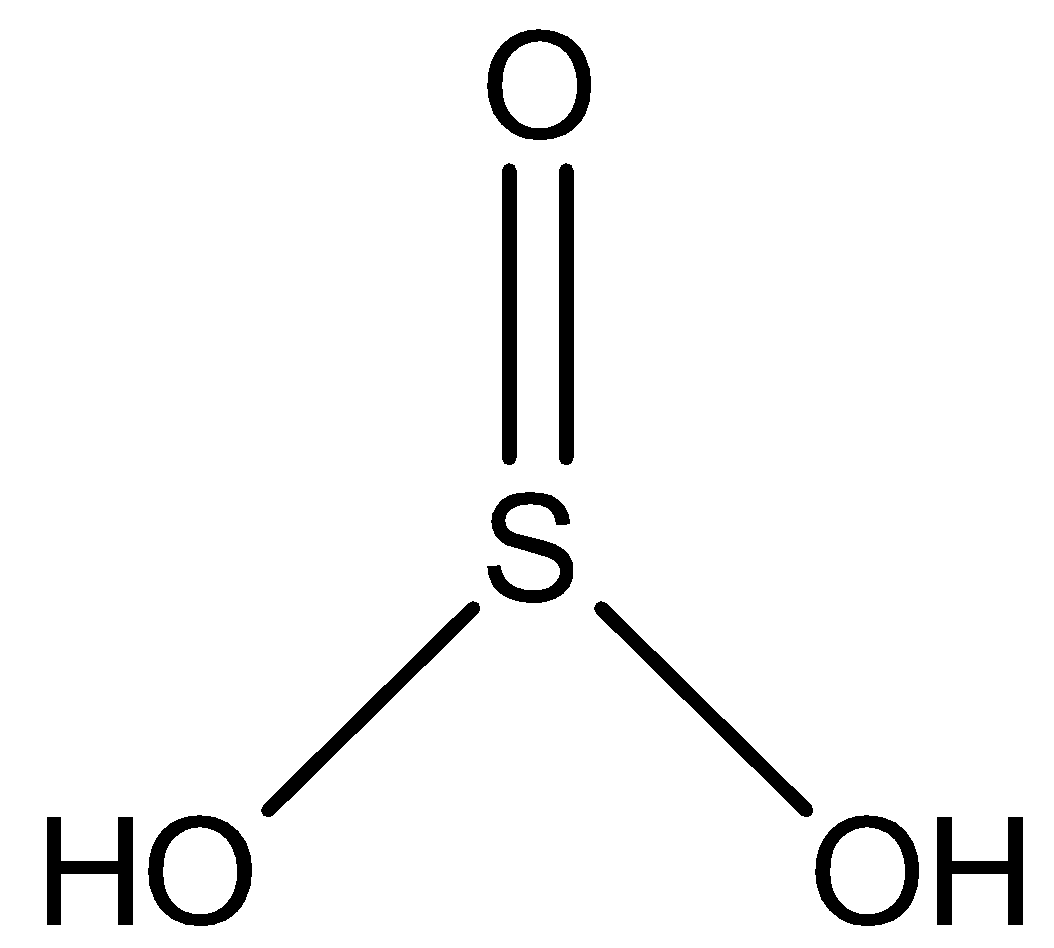
The equation of the sulfurous corrosive is given as ${H_2}S{O_3}$ . Where, the particle comprises two molecules of hydrogen, three iotas of oxygen and one iota of sulfur.
Taking a gander at the synthetic design of corrosive we can see that the particle has the sulfur iota in the center and three oxygen iotas are reinforced where two oxygen molecules are shaped by a solitary bond and one has a twofold bond. The two hydrogen molecules are associated by a solitary cling to the oxygen iotas. The sulfurous corrosive underlying equation is typically composed as-
Note:
We need to know that the sulfurous corrosive is a feeble and dibasic corrosive. It responds with bases to frame bisulfite and sulfite salts. Utilizations: Sulfurous corrosive and its salts are utilized as incredible lessening specialists and cleaning specialists. It is likewise utilized as a gentle blanching specialist for applications having chlorine delicate materials.
Complete answer:
We have to know that the sulfurous or sulfurous corrosive recipe ought not be mistaken for the equation of sulphuric corrosiveness. While the equation may appear to be comparable yet one of the principle contrasts is that there is less number of oxygen particles in sulfur corrosive. Their physical and synthetic properties additionally contrast partly. Sulfurous corrosive is commonly a dibasic or feeble corrosive and is a watery arrangement of water and sulfur dioxide. It is dry and has a sharp scent.
The equation of the sulfurous corrosive is given as ${H_2}S{O_3}$ . Where, the particle comprises two molecules of hydrogen, three iotas of oxygen and one iota of sulfur.
Taking a gander at the synthetic design of corrosive we can see that the particle has the sulfur iota in the center and three oxygen iotas are reinforced where two oxygen molecules are shaped by a solitary bond and one has a twofold bond. The two hydrogen molecules are associated by a solitary cling to the oxygen iotas. The sulfurous corrosive underlying equation is typically composed as-

Note:
We need to know that the sulfurous corrosive is a feeble and dibasic corrosive. It responds with bases to frame bisulfite and sulfite salts. Utilizations: Sulfurous corrosive and its salts are utilized as incredible lessening specialists and cleaning specialists. It is likewise utilized as a gentle blanching specialist for applications having chlorine delicate materials.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




