
What is the chemical name of vinegar?
Answer
513.3k+ views
Hint: Vinegar is an aqueous form of ethanoic acid and it is majorly used in food flavouring. It is available in the market in excess at an affordable price and is useful for remedies for individuals with diabetes. It is a major source of polyphenols and can be formed by the oxidation of fermentable carbohydrates which includes grapes, berries etc.
Complete answer: Vinegar is a dilute form of ethanoic acid which is also known as acetic acid. The chemical formula and molecular formula represents the proportion in which the atoms are present in a chemical compound. Therefore, the chemical formula of vinegar is similar to that of acetic acid i.e., $C{H_3}COOH$ or ${C_2}{H_4}O$.
In the structure of vinegar, it consists of one methyl group bonded to a carboxylic group via a single bond. Its structure is represented as follows:
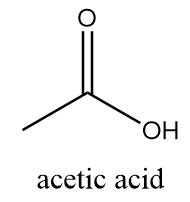
Hence, the chemical name of vinegar is acetic acid.
Additional information-
The chemical properties of vinegar are as follows:
1.It is a covalent compound which exists in a viscous liquid phase.
2.When it is heated, it decomposes to emit irritating fumes.
3.It is very flammable in nature.
4.It reacts with baking soda to form sodium acetate along with the removal of water and carbon dioxide.
Note:
It is important to note that according to the Food and drug administration i.e., FDA the vinegar must have a minimum of $5\% $ acidity that means in vinegar the percentage of acetic acid must lie between $5 - 8\% $. Also, vinegar is widely used in reducing systolic blood pressure and also helps in controlling blood sugar levels.
Complete answer: Vinegar is a dilute form of ethanoic acid which is also known as acetic acid. The chemical formula and molecular formula represents the proportion in which the atoms are present in a chemical compound. Therefore, the chemical formula of vinegar is similar to that of acetic acid i.e., $C{H_3}COOH$ or ${C_2}{H_4}O$.
In the structure of vinegar, it consists of one methyl group bonded to a carboxylic group via a single bond. Its structure is represented as follows:

Hence, the chemical name of vinegar is acetic acid.
Additional information-
The chemical properties of vinegar are as follows:
1.It is a covalent compound which exists in a viscous liquid phase.
2.When it is heated, it decomposes to emit irritating fumes.
3.It is very flammable in nature.
4.It reacts with baking soda to form sodium acetate along with the removal of water and carbon dioxide.
Note:
It is important to note that according to the Food and drug administration i.e., FDA the vinegar must have a minimum of $5\% $ acidity that means in vinegar the percentage of acetic acid must lie between $5 - 8\% $. Also, vinegar is widely used in reducing systolic blood pressure and also helps in controlling blood sugar levels.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




