
What is silicone made of?
Answer
509.7k+ views
Hint: We need to know that the silicone is also known as polysiloxane is a colorless synthetic polymer and it is a rubber - like or oil - like substance which is prepared by repeating chemical units and is known as the monomer. This monomer is very small that is attached to form a long chain. And silicone is used in many areas as it is used in medicine, cooking utensils, sealants, lubricants, and electrical and thermal insulations, etc. Silicone is a mix of chemical additives which is obtained from fossil fuels.
Complete answer:
We need to remember that the silicone is a synthetic polymer and it is made up of siloxane with a chemical formula, \[ - {R_2}Si - O - Si{R_2} - \], the R is an organic functional group. The silicone contains repeating units of carbon atoms and the main chain of the silicone is polysiloxane which is made up of silicon and oxygen backbone having the side chain hydrocarbon or hydrogen groups linked with these silicon atoms. In silicone, each of the silicon makes a bond with substitution groups which are two carbon - based groups, always its methyl groups. Let’s see the structure of silicones,
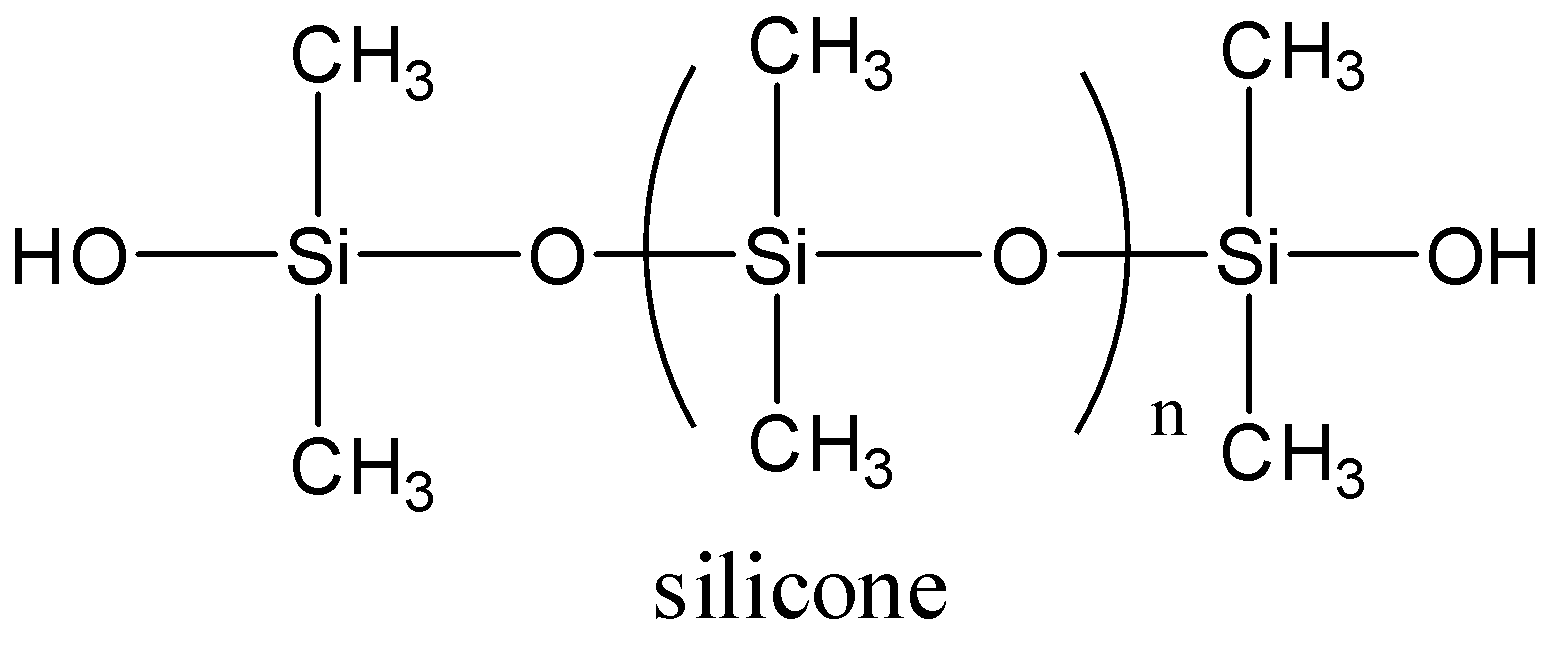
Note:
We have to know that the silicone is an inorganic polymer which is made up of siloxane. In this polymer, each silicon atom is linked with substituent groups. Silicone is a chemically stable compound and its use may cause toxicity. And it forms harmful materials while burning. Silicone is considered plastic in the plastic industry. And it is used to prepare rubber- like items, resins, etc.
Complete answer:
We need to remember that the silicone is a synthetic polymer and it is made up of siloxane with a chemical formula, \[ - {R_2}Si - O - Si{R_2} - \], the R is an organic functional group. The silicone contains repeating units of carbon atoms and the main chain of the silicone is polysiloxane which is made up of silicon and oxygen backbone having the side chain hydrocarbon or hydrogen groups linked with these silicon atoms. In silicone, each of the silicon makes a bond with substitution groups which are two carbon - based groups, always its methyl groups. Let’s see the structure of silicones,

Note:
We have to know that the silicone is an inorganic polymer which is made up of siloxane. In this polymer, each silicon atom is linked with substituent groups. Silicone is a chemically stable compound and its use may cause toxicity. And it forms harmful materials while burning. Silicone is considered plastic in the plastic industry. And it is used to prepare rubber- like items, resins, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




